Husbandry Manual for Asian
Lorisines
(Nycticebus & Loris ssp.)
HABITAT DESIGN
.
Minimum
Standards
for Housing Asian Lorisines
(Adapted from AZA guidelines for
the
Family Lorisidae)
Contributed by Lisa Bottcher-Law
When developing spaces for lorises,
every
consideration should be made to enhance and maximize their use of
that
space. If space available is of a lesser quality—possibly because
it is
temporary housing or there are other extenuating
circumstances—here are
some minimum guidelines.
Temperature: 65½ F (18½ C) min. - 85½ F (30½
C) max.
In warmer climates where outdoor access is possible, heated
nest boxes
and/or indoor access (which are kept within the above range)
should be
provided. Providing a temperature gradient is always optimal. It
allows
a variety of options for this taxa, whose thermoregulatory
capabilities
are primitive.
Lighting
The lighting requirement is
approximately
12 hrs/day. Unless they are outdoors in natural lighting, full
spectrum
illumination is suggested (a minimum of 75fc is required,
(Keeling, 1974)).
A dimming feature to simulate dawn/dusk is preferable.
Moonlighting should
be done with neutral density acetate filters. It has been
suggested that
a blue filter may be perceived just as white light is to the
prosimian
eye (Frederick and Fernandes, 1994). It follows, that if neutral
density
is not available, red light would be a better choice.
.
Ventilation
In indoor situations with
non-recirculated
air, 15 changes per hour are recommended (Keeling, 1974). Where
possible,
keeper/public areas should be ventilated separate to the animal
areas.
.
Humidity
Relative humidity should be
maintained
between 40% and 60%. Substrate on floor (i.e. leaves and chipped
wood)
help maintain humidity if cage is misted daily.
.
Exhibit/Cage Size
This space should allow the loris
to be
able to meet its need to have solitary foraging and to locomote
easily
from branch to branch, as they will not leap. Maximizing surface
area by
providing many pathways of varying sizes (1/2"-4" diameter) and
making
visual barriers will make the most of any space. At least one nest
box
should be provided (no smaller than 12"1 x 4"w x6"h). They do like
to vary
sleep sites, so leafy cover in branches and boxes on the floor can
also
provide other options. Exhibit dimensions for this group should be
no smaller
than 2.5x2.5x2.5m. This is the minimum and not the optimum, so
when new
spaces are being designed more space should be considered.
.
Social Grouping
In the wild, male home ranges
overlap
with several females. Optimally, separate cages that individuals
can rotate
into when they cannot be housed together (e.g. situation where
breeding
should be avoided) is preferable. Lorises are usually best housed
as a
breeding pair or mother with
71
 Loris
Husbandry Manual
Loris
Husbandry Manual
immature offspring. If an individual
has
to be separated for any length of time (for medical or breeding
reasons),
visual and olfactory access should be allowed. Lorises have been
shown
to do well in same sex and extended family groups, but individual
situations
(aggression) may not allow this. Lorises are solitary by nature,
but they
are NOT asocial. Maximizing space by housing multiple individuals
together
allows individuals more stimulation. Shared space is usually
larger than
individual space, and the animals are able to interact with their
cohorts.
Social housing also allows zoos to free up much-needed nocturnal
prosimian
space.
General
Habitat
Design
The cage design needs to fulfill a
variety
of animal and keeper needs. Because lorises will not jump from one
branch
to another, climbing structures need to be close enough to each
other to
provide a continuous pathway. The animals should be able to reach
one branch
while situated on another. Vertical trunks and branches with large
diameters
do not provide adequate climbing structures for lorises (Schulze,
1998).
Dangerous falls can result when the animals are unable to maintain
their
grip on such surfaces. Thus horizontal branches, wire mesh, and
lianas
provide much more suitable climbing and clinging surfaces.
It is important to also consider
keeper
access when designing the perching network. When handling is
necessary,
dense vegetation requires tactics such as baiting or timing
captures when
the lorises are asleep in their nest boxes. Chasing them in dense
vegetation
is destructive to the enclosure and very stressful to the lorises.
Plant foliage and nest boxes will
provide
cover and sleeping sites. Some lorises change sleeping sites
frequently,
so several nests should be available. A removable nest box that is
situated
in an easily accessed area is advantageous for handling or
capture. The
whole box can be removed while the loris is contained inside. The
nest
box should have a smooth surface so that the animal can easily be
removed
without obtaining a grip on the box.
The least stressful method of
separating
lorises is to provide a passage or tunnel between two adjacent
cages. An
animal can be coaxed through the tunnel into the other cage while
a gate
is closed behind the loris. The keeper should be able to view both
cages
and the tunnel simultaneously while operating the gate from the
outside.
Horizontal branches are especially
important
for breeding purposes, because copulation usually takes place in a
suspended
position from a horizontal branch. Additionally, most behavioral
postures
are exhibited preferentially on the horizontal branches (Glassman
and Wells,
1984). When given a choice, lorises prefer to occupy the higher
elevations
of the enclosure. They usually flee upwards when threatened by
other than
conspecifics. Cover at low levels in the cage provides animals
with escape
from aggressive cage mates and may facilitate avoidance of
agonistic encounters.
If the lorises are in public view, it
is
additionally important to provide adequate cover. The animals
should have
enough foliage to feel secure and comfortable moving around,
without becoming
invisible to zoo visitors. Open areas can be provided toward the
middle
of the enclosure so that the public can look through a foreground
of vegetation
to open areas with water or food sources. Food bowls should not be
situated
too close to the ground, since some lorises may be reluctant to
72
Habitat design
climb to lower levels if they are shy or stressed. It is also a
good
idea to offer food in several areas of the enclosure to provide an
enriched
environment (see Enrichment section).
A shelf above the food dishes
will prevent urine and feces from falling into the food from
above.
.
In exhibit areas, a small waterfall
or
pool of water may be added to make the enclosure more attractive
and humidify
the air. However, lorises will not voluntarily go into the water,
and there
may be a drowning risk. A slender loris juvenile in Germany was
found dead
with its head in a pool created by a waterfall, although the exact
cause
of death was not determined.
.
Slender lorises are easily upset
during
transfers, and this may result in mortality. Environmental
enrichment and
special attention to housing conditions the first days after
transfer can
minimize stress. This can be accomplished by covering the cage
fronts with
blankets, minimizing disturbances, and offering live insects to
encourage
food intake.
.
Daschbach, Schein, and Haines
(1982/1983)
conducted an experiment at West Virginia University to investigate
the
behavioral effects of cage size on slow lorises. Two male-female
pairs
were switched between a 0.42 cubic meter and a 8.75 cubic meter
cage, and
social behaviors did not differ significantly between the two cage
sizes.
Animals kept in the larger cage locomoted significantly more, and
residue
accumulation was much heavier in the smaller cage. This resulted
in poorer
conditions of the lorises’ coats, which were tacky to the touch.
The lorises
in the larger cage were observed to roll in sawdust on the floor.
When
the animals in the smaller cage were allowed access to sawdust
their coat
conditions improved. (However, it should be noted that sawdust may
cause
respiratory problems in lorises.) The study concluded that lorises
can
be kept together in relatively small cages but a decrease in
activity will
result. Psychological effects of small cage size are more
difficult to
determine. An optimum environment provides enough space and
enrichment
for an active, healthy lifestyle.
73
 Loris
Husbandry Manual
Loris
Husbandry Manual
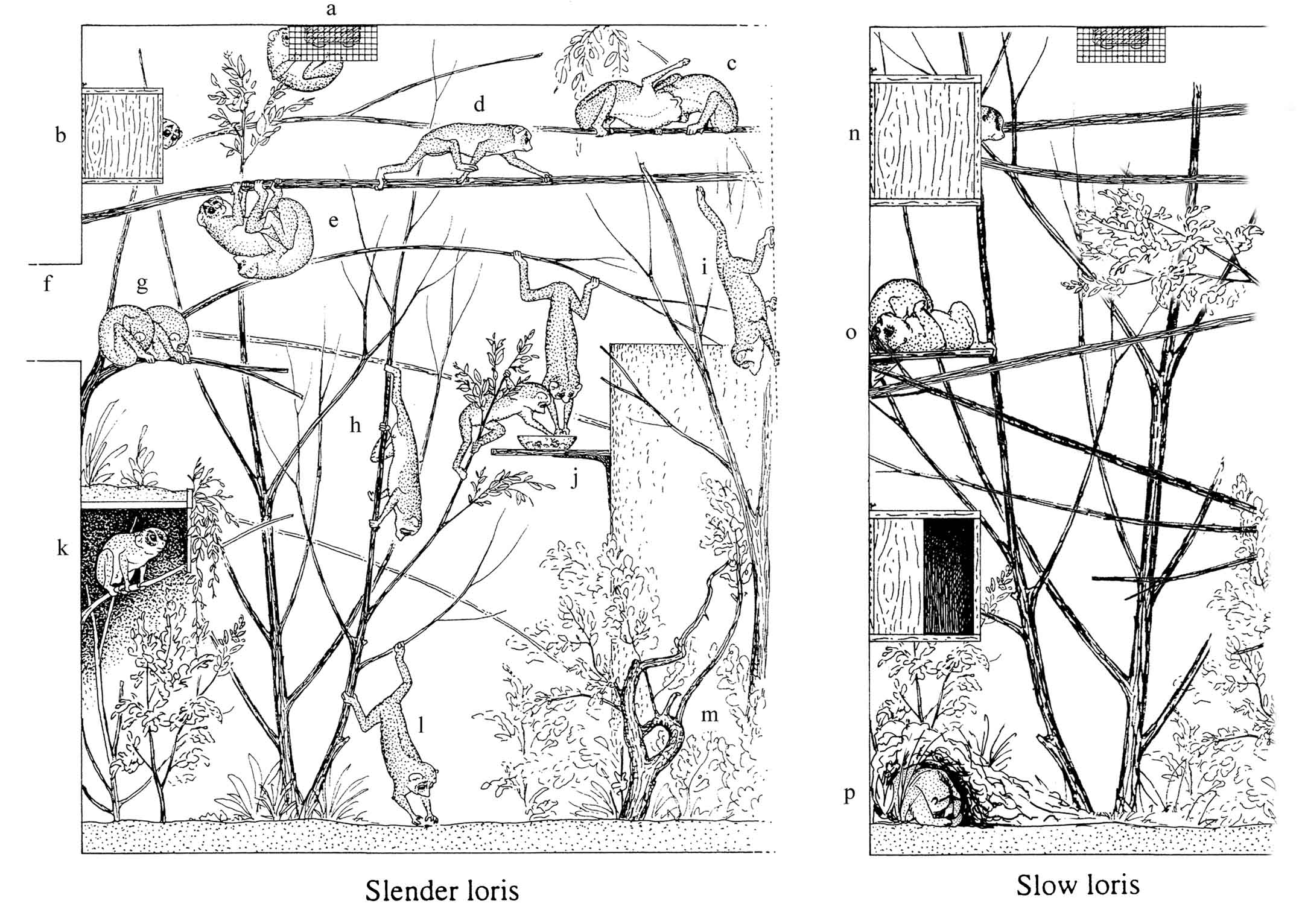
Figure 29: Examples for the
use of
adequately furnished cages (see page 74). The figure does not show
normal
population density of the species. (Figure from: Schulze 1998).
Left:
Loris tardigradus.
If lorises are disturbed or frightened, they usually go up as high
as possible,
for instance clinging to a wiremesh ceiling in hanging posture
behind some
cover (a). Some animals prefer to hide in boxes (b)
when
disturbed, some like to sleep in boxes or hidden between leaves,
but often
Loris
sleep
in the open. Energy-saving stay on top of horizontal branches is
characteristic
for most Loridae behavior, particularly for resting and comfort
behavior
(c: allogrooming). d: Longer horizontal branches
in Loris
encourage
a fast, trot-like locomotion which is seldom seen on irregular
substrate.
e:
Substrates
in the upper part of the cage allowing hanging postures (thin
horizontal
branches, wiremesh) are important for sexual behavior.
f:
Passages
to neighbouring cages have several advantages: they allow easy
separation
of animals, cleaning of cages without animals inside, they are
frequently
used by the animals for having a look at the environment and
considerably
promote locomotor activity. g: For sleeping, places with
a lateral
support are appreciated.
h:
Vertical substrate is used for climbing
up and down. i: Wiremesh, both horizontal and vertical,
is a valuable
substrate for climbing. Wiremesh walls can help to assure
continuous locomotor
opportunities without “dead end” branches.
j:
Food ought to be offered
in an elevated place; for shy animals, some cover close to the
feeding
places is helpful. k:
In cases of quarreling, frightened animals
go down and try to hide; shelters protecting the refugee against
sight
from above can diminish social stress. Such hideaways ought to
have a second
exit allowing escape from aggressive conspecifics.
l:
live insects
on the floor promote climbing and hunting. m:
The use of the lower
parts of cages can be improved by undergrowth.
Right: Additional
recommendations for
Nycticebus
cages.
In
both slow loris species, sleeping boxes (n), tubes, or
other hollow
hideaways or dense plant cover around comfortable sleeping
branches are
apparently more important than in Loris. Boxes must be
large enough
for the group; additional boxes in lower parts of the cage or even
on the
ground have been readily used by some groups under normal
conditions, with
no
74
Habitat design
evidence of social stress (Fitch-Snyder; Schweigert, pers. comm.;
Lippe,
pers. comm.). If the temperature is very warm, N. coucang at
the
San Diego Zoo like to sleep flat on their backs on a shelf (o);
in
N. pygmaeus, lying on the back while sleeping has not been
observed
(Fitch-Snyder).
N. coucang in the wild have been found walking on
the ground or sleeping hidden under leaves on the ground (Wiens,
pers.
comm.); in captivity, sleeping on the cage floor, hidden under
newspaper,
straw, towels, or other available substrate (p) also occurs
in N.
coucang. N. pygmaeus at the San Diego Zoo prefer
sleeping on
top of straw and in higher parts of the cage, but in other
facilities they
also regularly sleep on the floor, in boxes, or hidden under
substrates
(Fitch-Snyder; Schweigert, pers. comm.). In N. pygmaeus,
post-sleeping
period cases of hypothermia (cold body, abnormal movements and
equilibrium
problems or animal even lying on the ground, showing little
reaction) have
occurred. A sufficiently high temperature for all potential
sleeping places
(including the floor) seems necessary (Schweigert, Lippe, pers.
comm.).
Left figure: with permission by International Zoo Yearbook.
.
75
 Loris
Husbandry Manual
.
Loris
Husbandry Manual
.
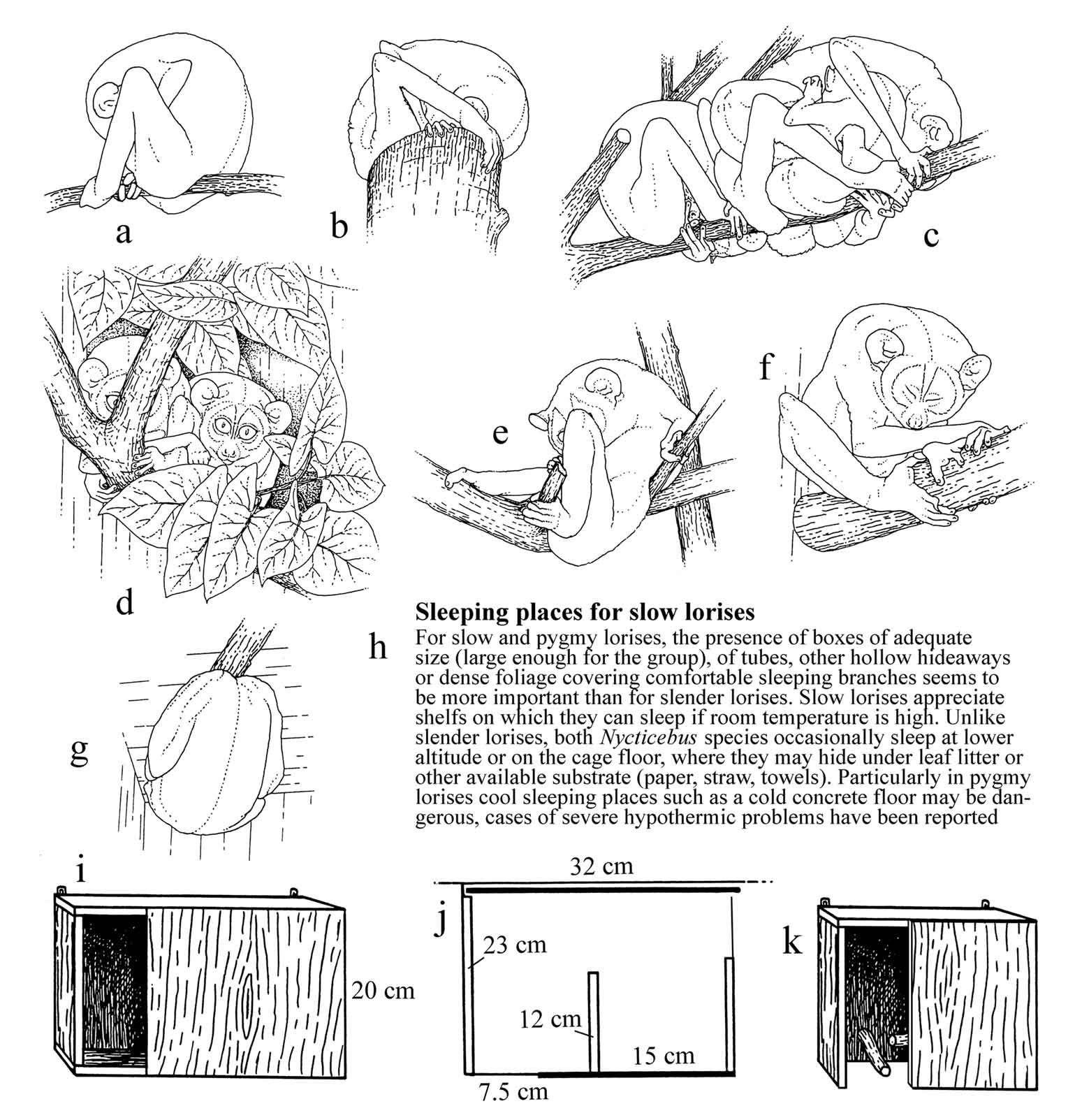
Figure 30: Choice of sleeping
places
and meaning of different sleeping postures (behavioral examples: L.
t.
nordicus).
Slender lorises usually sleep in the
upper
parts of their cage, sitting on branches (a, c) or
on horizontal
surfaces (b; for slow lorises, see also Figure 29 o).
Sleeping
in the open is common in animals that feel safe. Sleeping places
providing
some cover and lateral support (d), however, are
appreciated. Cover
as shown in (d) or sleeping boxes are particularly
preferred by
shy animals or in periods of environmental distrubance. Sleeping
huddled
together (c) is common. If animals formerly sleeping
together suddenly
choose distant sleeping places, social stress after quarreling may
be a
reason. Sleeping on branch forks in the lying posture is sometimes
shown
by youngsters still used to sleeping cradled on their mother’s
legs (e),
as an individual habit or in sick and old animals as a sign of
weakness.
When ambient temperatures are high, the animals often do not roll
up tightly
as usual, but in relaxed postures more adequate for emitting heat
(f).
Hanging under the ceiling of the cage during sleeping periods (g)
is usually a sign of environmental distress; it shows flight
tendency upwards
where the animal feels safest. The text under (h) mentions
different
needs in the Nycticebus species. Warm sleeping places for N.
pygmaeus
may
prevent hypothermic problems in cool rooms. Slender lorises,
particularly
old and sick animals, may need a warm place for sleeping. (i)
and
(j) show a sleeping box as used at Ruhr-University, size
adequate
for slender lorises, with a second lateral exit added for escape
in cases
of quarreling. (j): interior seen from above, with a small
median
wall providing additional opportunity to hide; the back wall can
be removed
for cleaning. The box might be further improved by branches inside
as in
the second proposed type of sleeping box (k); no preference
test
for different boxes has been made so far at Ruhr-University. Slow
lorises
would of course need larger boxes, sufficient for several animals.
76
Habitat design
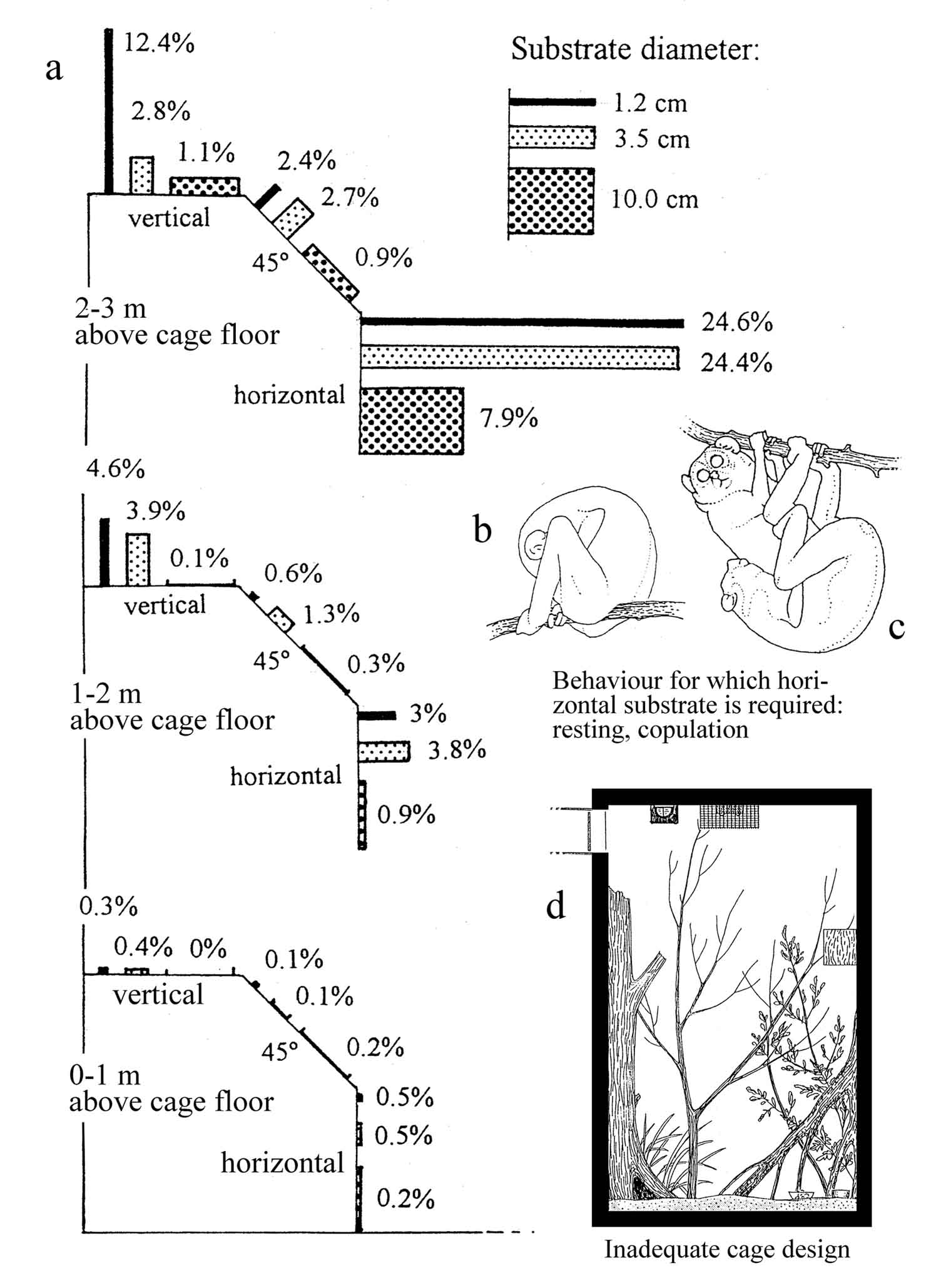
Figure 31: Substrate use
during activity
period by captive slender loris.
In captive breeding of lorises,
substrate
qualities play an important role.
a: substrate utilization in a
cage furnished with equal amounts of smooth round timber perches
of the
following types: diameters 1.2 cm, 3.5 cm and 10 cm, each offered
in three
altitudes and three inclinations. (Data by G. Hoeschen; 10
animals; n =
5527 observations = 100%). Under normal conditions, animals show a
clear
preference for the upper part of the cage (under conditions of
social stress,
inferior animals would tend to stay at lower altitudes). Small
diameters
allow a safe grip with the rather small hands and are therefore
preferred,
especially in vertical substrate. Horizontal perches allow
energy-saving
locomotion and postures (on top of the substrate or in hanging
posture).
The highly preferred thin horizontal perches in the upper part of
the cage
are particularly important for resting and copulatory behavior;
low breeding
success in some zoos might be due to a lack of such substrates.
77
 Loris
Husbandry Manual
.
.
Loris
Husbandry Manual
.
.Lorises kept in outside enclosures
may
require additional heating during cooler months. Slow lorises at
San Diego
Zoo have heat lamps and large heated nest boxes. Although outdoor
temperatures
can drop below 5°C (40°F) during the coldest season, slow lorises
remain fairly active as long as these heat sources are available.
.
Slender and pygmy lorises are less
tolerant
of low temperatures than slow lorises. Lowland slender lorises,
with their
slender limbs and large ears, are more adapted to high
temperatures than
to cold exposure. Healthy L. t. nordicus withstood a
temperature
of 16-17½C(60-61½F) for some time. During cold exposure,
in Loris only a rather small body core is kept warm,
whereas large
parts of the body are allowed to cool (Müller et al., 1985). Two
old
L.
t. nordicus showed equilibrium problems immediately after
sleeping
in a cooler room. The mountain form L. t. nycticeboides with
its
thick fur is adapted to rain and mist forests where temperatures
may fall
below 0½C. Loris infants have very thin fur and can easily become
hypothermic. Slender lorises suffer less from heat than the other
lorises
because they can increase heat emission over their slender limbs
and large
ears. They show reduced activity and energy-saving behavior that,
under
normal circumstances, would be regarded as signs of weakness or
disease.
They do not roll up as usual and sometimes sleep laying down. They
sleep
with their faces visible, often hanging with their limbs down.
They apparently
emit heat from enlarged veins in the ears and, in males,
over-enlarged
testes.
.
Pygmy lorises are more tolerant of
cold
environments than slender lorises, but they do not adjust as well
as slow
lorises. For example, in cold weather, pygmy lorises may not
venture out
of a warm nest box to feed if food items are not within their
reach. Conversely,
they may sleep in the coldest areas of their enclosure instead of
seeking
the warmth of their heat lamp or nest box. In pygmy lorises, a
hypothermic
condition with locomotor problems to unconsciousness has been
reported
after sleeping in cool places (Schweigert, pers. comm.; Lippe,
pers. comm.).
.
In general, lorises appear to be
most
comfortable in temperatures around the mid 20s C (mid 70s F)
during the
day and low 20s C at night (high 60s F).
Animals modify their activity rhythms
according
to the light and dark phases of the day, and environmental factors
modify
their “internal clocks.” Loris activity usually begins at dusk,
which changes
seasonally. Dim light cues influencing emergence can be overridden
by meteorological
and physical factors such as rain, heat, or hunger (Kavanau and
Peters,
1976).
A study of slow lorises under an
experimentally
varied light regime showed that their locomotor activity became
synchronized
to the period of darkness. When the conditions were continuously
dark,
an endogenous cycle of less than 24 hours emerged. The slow
lorises drifted
out of phase with the outside world. Animals housed together
synchronized
their schedules (Redman, 1979).
Kavanau and Peters (1979) found that
arboreal,
nocturnal primates are not well adapted for activity in complete
darkness.
Illuminance preference was assessed by allowing subjects to
control the
ambient light level and by assessing activities in imposed light
conditions.
Slow lorises were found
78
Habitat design
to be adapted for a relatively broad range of night illuminance
(0.007
to 0.19 lux). They appear to prefer a mid-dim range of 0.07 to
0.19 lux.
Complete darkness and very bright light could severely inhibit
activity.
Another study suggested that the activity level is a function of
illumination.
Moonlight illumination resulted in low activity behaviors while
twilight
intensities resulted in high activity behaviors (Trent, et al.,
1977).
.
In captivity, reversed light cycles
are
often used, especially when the animals are exhibited to the
public. The
reversion is also preferred when caretakers work regular hours
because
it is difficult to observe the animals’ condition and recognize
unusual
behavior as they sleep. Night lighting is simulated with dim red
lights
while bright white lights are used for the simulated day.
Incandescent
lights with acetate filters are a natural-looking alternative.
.
Frederick and Fernandes (1994)
studied
a nocturnal exhibit at the Franklin Park Zoo that housed
Perodicticus.
The potto is the African “equivalent” of the slow loris. The
animals were
inactive and usually out of sight of the viewing public. After the
lighting
scheme was modified, the result was a dramatic increase of the
activity
level and exhibit use.
.
The lighting directly outside the
exhibit
was reduced, which decreased glare and level of contrast between
the light
inside and outside the exhibit. The “day” lighting was increased,
which
caused activity to be displaced to “night.” The “night” lighting
was changed
from blue fluorescent lights to track lights with white
incandescent bulbs.
The bulbs were covered by a .6 neutral density acetate filter that
removes
75% of the light. By covering the light source with an acetate
filter,
the visible portion of the spectrum was filtered evenly and the
ultra-violet
light was significantly reduced.
.
Frequent cage cleaning is not
usually
recommended. Lorises (especially the slender lorises) can become
stressed
by cleaning procedures. Keeping the animals in two cages that
allow separation
may minimize the stress. The animals can be moved to one cage
while the
other is being cleaned.
.
Loris feces are small, dry, and do
not
require prompt removal because they do not have a strong scent.
Washed
off urine will promptly be replaced because it serves as territory
marker.
However, too much urine build-up can irritate skin, and branches
should
be replaced or washed every few weeks. Some zoos clean the cages
relatively
often and their lorises have become habituated to the procedure.
Slender
loris cages stay cleaner longer than slow and pygmy loris cages
because
the build up of body secretions occurs at a faster rate among the
larger
species (Weisenseel, 1986).
.
When using leaf litter (refer to
Environmental
Enrichment section) it should be loosened or “fluffed” to provide
a fresh
area for the loris to traverse. This should be on a daily basis.
The leaves
and branches above should be misted with a hose to rinse away food
residue,
dilute scent-marking trails, and increase humidity in the
enclosure. A
super-fine misting system that intermittently sprays tepid water
from above
can be used to recreate the rain forest experience. A mist fine
enough
to dissipate before reaching the floor is best. The floor
substrate should
be allowed to dry out on a daily basis; if kept wet, the fallen
food/feces
can become moldy overnight. Earwigs and earthworms may exist in
the leaf
litter but seem to cause no problems; earwigs will consume feces
and fallen
food items. Intermittently, the substrate should be completely
removed
and the enclosure disinfected. The timing
.
79
 Loris
Husbandry Manual
Loris
Husbandry Manual
of this is dependent on the size of the enclosure and the number
of
animals. At Woodland Park Zoo, this is done every three to four
weeks.
Between cleanings, fresh mulch is added to give the lorises new
olfactory
stimulation.
.
Washable enclosures should be
cleaned
with water and a disinfectant. Hot water should be used cautiously
because
it aerosolizes microorganisms. It is important to rinse well
because detergent
residues can otherwise cause skin lesions. The branches and vines
should
be replaced as they are chewed by the lorises. Floors that are not
covered
with bedding should be hosed off daily.
.
Food dishes should be removable and
mounted
on washable surfaces. Surrounding branches may become caked with
formula
and sticky food residues. A solid wire mesh hanging over the food
can instead
give access to the food. The wire mesh should be constructed so it
can
effortlessly be removed for cleaning. Another solution is to
provide smaller
twigs that can easily be replaced.
.
Contributed by Lisa Bottcher-Law
.
Environmental enrichment for
lorises,
as for any captive species, consists of providing conditions that
are as
similar as possible to their natural habitat. Exhibit design and
enrichment
are paramount in providing a loris with a stimulating environment
that
results in an active lifestyle.
.
Lorises are found in dense tropical
forests
that are characterized by high humidity and fairly stable
temperatures.
These conditions allow for a high diversity of densely growing
vegetation.
For this reason, lorises feel most comfortable with lots of cover.
A variety
of branch widths (1”-4” diameter) both vertically and horizontally
placed
is best for the basic framework. Branches should be placed so as
to maximize
the use of the whole exhibit. No zoo exhibit comes close to the
size of
their natural home ranges, so it is important to make the most of
the space
that is available.
.
Branches
.
At Woodland Park Zoo, plastic cable
ties
are threaded through pre-drilled holes at the ends of branches for
easy
installation. Lorises will use a variety of widths of horizontally
placed
branches, but the limited enclosure dimensions allotted for most
lorises
will preclude use of anything larger than 3”-4” diameter. Once the
larger
branches have been installed, interconnecting all the major areas
of the
enclosure, smaller branches with lots of leaves can be placed
vertically.
These smaller branches can be attached to the horizontal branches,
again
with the use of plastic cable ties. (See Table 29 for list of
plants.)
Any type of leafy branch that is non-toxic will do, but some with
good
leaf retention (when dry) include birch, bamboo, and camellia. As
always,
if the climate is warm enough to keep them outside, using live
plants that
can be rotated when they become “worn” is best. Few locales meet
these
climate requirements, and most zoos will be dealing with indoor
nocturnal
enclosures. One big advantage of this is that dried leaves look
good under
artificial moonlight.
Attaching branches so that there is
some
movement is always more naturalistic than runs of rigid ones. This
allows
the loris to become more adept at negotiating its surroundings.
Hanging
smaller width branches (1”-2”) vertically, with only one
attachment at
the top and an open space around to allow rotation, makes for an
interesting
pathway for these prosimians. Lorises may be insecure about
.
80
Habitat design
climbing on branches that swing freely, so it may be necessary to
bait
the branches with food to encourage exploration. Once they became
familiar
with the swinging branches, the lorises at Woodland Park Zoo used
them
exclusively.
The use of leafy vegetation
provides the
loris with visual barriers to the public. Visual barriers (any
item natural
or unnatural that blocks a view) can also provide the animal with
more
interest and the sense that their enclosure is larger than it
appears.
Without the ability to see from one end of its enclosure to the
other,
the loris is encouraged to explore its environment.
Surreptitiously hiding
items all around the enclosure will increase the loris’
foraging/exploration
time. They will check areas that have previously had food even if
no food
has been present for some time.
.
Floor substrate
.Lorises are arboreal primates but
are
known to come to the ground and even to sleep in leaf litter. With
the
small home range that lorises are allocated in captivity, optimal
use of
floor space can increase useable surface area. A natural way to
achieve
this is to cover the floor with a bottom layer of chipped wood. At
Woodland
Park Zoo, Horticultural staff supplies trees/shrubs that have been
through
the chipper. Some tree services will drop off their mulch for
free, but
it is important to be sure that no pesticides or toxic plants have
been
used. With a good 2” layer of wood mulch on the bottom, a layer of
leaf
litter (1-2”) on top can be added using fallen deciduous leaves.
Even leaves,
such as oak, that are toxic when green lose this toxicity once
they have
fallen. Leaves can be collected year round. Even if collected when
wet,
they can be stored in plastic bags for many weeks, as long as the
bags
are left wide open to air out. The natural decomposition that
takes place
in this natural floor strata does an amazing job of cutting down
the loris
odor. Fresh mulch/leaf litter can be added to give them new
olfactory stimulation.
Lorises will scent mark all over the fresh leaves.
.
Hide areas
.
In addition to the conventional
nest boxes
that are hung in the upper portions of the enclosure, lorises will
choose
boxes provided on the floor. Thick, four-inch diameter cardboard
tubes
can also be used. Boxes as large as an inverted apple box can
provide a
good hide area. Cylindrical oatmeal containers make for great
entry portals.
Cutting flaps into various areas in the box easily provide various
entries/exits.
This is important to allow an animal an easy exit if being pursued
by another,
most commonly in a mating situation.
Boxes are usually only acceptable
when
a naturalistic look is not as important, such as in holding areas.
However,
even a plain brown cardboard box, carefully concealed with mulch,
tall
grass, or leaves, can disappear into the surroundings. The same
can be
done with larger (6”) PVC tubes that are painted a natural color.
By using
floor space to create new spaces (rooms), the usable surface area
is increased,
giving extra sleeping sites and a place to hide food items.
Lorises appear
to spend more time on the ground if there are:
.
* Several perching pathways that
lead
to the ground.
* Substrate on the floor
(encourages them
to scent mark and hunt for food).
* Grass clumps, boxes, tubes, any
structure
to allow hiding places as they travel on the floor.
.
81
 Loris
Husbandry Manual
Loris
Husbandry Manual
By providing lorises with a varied dense and leafy enclosure, many
behavioral needs can be met. Besides having a variety of pathways
to locomote
through, the visual barriers created by dense foliage or other
structural
barriers give the appearance of a larger space. The novelty of
discovering
food items found on a branch in a unseen area has many benefits.
As David
Shepherdson, Ph.D pointed out in his seminar at Woodland Park Zoo
(January
1993), “ The ability to provide an animal with the control over
his environment
is very important for captive species.” Lorises are provided a
level of
this control by being able to hide deep in the leafy branches when
threatened,
and being able to explore new, unseen areas in their environment.
Ideally,
lorises would be foraging independently and intermittently cross
paths
with other lorises where ranges overlap. This natural model is not
possible
to duplicate when individuals must share the same small space. But
with
the presence of visual barriers, several sleeping areas, food
distributed
throughout the branches, and leaf litter, captive lorises can have
a semblance
of their natural routine to potentially allow them to retain their
unique
adaptations through generations of captive life.
.
Environmental Enrichment: A
Holistic
View
.
Environmental enrichment is a term
that
has become very popular in the zoo world in the past few years. It
is a
very necessary progression in the welfare of captive species, but
it suggests,
through the word enrichment, that we are providing an abundance or
at least
an ample amount of stimulation relative to the wild situation.
Captive
life, in the best of circumstances, only scrapes the surface at
providing
even the basic number of variables necessary to keep a species
phenotypically
correct over an individual’s lifetime or any number of
generations. Some
of these variables include climatic adaptability, foraging
technique, competition,
predation, and mate selection. The importance of these
interactions has
become abundantly clear as more field information is gained about
species
in their natural ecosystems, and as the interrelatedness and
interdependence
of multiple organism is better understood. The challenge for zoos,
therefore,
is to gain as much natural history information as possible on a
species
held in captivity and then try to mimic that scenario as closely
as possible.
In general, the goals for enrichment should be to provide as many
variables
as possible so that species/ individuals can develop and maintain
natural
behavioral repertoires. In return, the needs of the zoo can be
more easily
met. Increased natural activity will serve to entertain and
educate the
public, while increased reproduction will be more likely.
Therefore, environmental
enrichment goes far beyond providing toys for animals to play with
in an
enclosure. A holistic viewpoint should be taken, one that includes
factors
such as naturalized exhibit design, social structure, climatic
variability,
and foraging strategy.
.
The Natural Model
.
Conditions in the native habitat
provide
the basic model upon which species-specific enrichment should be
based.
Enrichment should be an integral part of lorisine husbandry - not
an “if
time allows” situation. In fact enrichment should be looked upon
as improved
husbandry. It can be done with some ingenuity and not much extra
effort.
The benefits to the animals and the viewing public will be readily
apparent.
.
Lorisines have special requirements
that
are most easily discovered by looking to life history; although
due to
the difficult nature of field study (dense forest, nocturnal
activity,
government, and access to in situ sites), information is
presently
sparse. As field methodology improves and findings
82
Habitat design
become available, that knowledge can be applied to captive loris
environs.
The more closely we can mimic the natural situation, the more
likely we
are to be able to retain the specific characteristics unique to
each lorisine
species. For example, there are specific reasons why N.
coucang and
N.
pygmaeus are able to occupy the same geographic locations.
Knowing
in detail the different niche requirements for each species and
trying
to mimic those in captivity can only benefit the goal of
maintaining diversity
in a captive group.
.
Keeping a log
.
Enrichment must be dynamic in order
to
try to mimic the barrage of stimuli the wild loris would encounter
on a
daily basis. For this reason, enrichment must occur at many
different levels,
such as, physical environment, social environment, food, and
sensory (Shepherdson,
1993). Variety is the basis for captive enrichment, and
maintaining a log
can simplify the process (see Table 28). The simpler the log is to
use,
the more likely it is that keepers will keep it up to date. Over
time,
it is important to have good records in order to remember all the
enrichment
items that have been tried, which animals were involved, and, in
brief,
their responses. If the behaviors surrounding an enrichment are
more involved,
detailed information can be written on the animal’s individual
record.
An enrichment log should be your quick reference catalog of
activities.
.
Table 28: Enrichment Log
|
Species
|
Animal
|
Date
|
Activity
|
Reaction Summary
|
|
N. pygmaeus
|
930331
|
35034
|
goldfish in water
|
grabbed at fish, picked
romaine leaves
looking for them; bit them but would not eat
|
|
N. coucang
|
890716
|
35036
|
willow treat log
|
Licked gum acacia, did
not chew wood
(due to gingivitis?)
|
By promoting natural activities with
stimulating
additions to the loris habitat, the conservation of these animals
is improved.
Reproduction, parent-rearing, normal social interactions, and
speciesspecific
behaviors are all enhanced by enrichment. These improvements are
partly
due to the increased physical well-being of individuals. More
exercise
and stimulation lead to proper physical and mental development.
Stimulating all the senses
There are many ways to simulate the
“wilds”
in captivity. As mentioned in the Housing chapter, climbing
structures,
visual barriers, substrate, and appropriate humidity are the basic
framework.
Auditory stimulation through the use of native nocturnal sounds
adds another
layer to filling in the complete environmental picture. A
repeating CD
or tape provides constant stimulation, which can help to submerge
the public
in the naturalistic experience and may drown out some of the
unnatural
human vocalizations for the lorises.
Olfaction is very important to this
intermittently
solitary species. Every loris begins its evening with a round of
scent-marking,
slowly dragging its hind end on the appropriate pathways. Several
things
can be done to encourage this important territorial behavior.
Misting the
cage allows some of the
83
 Loris
Husbandry Manual
Loris
Husbandry Manual
previous night’s scent-marking to be slightly diluted as it may be
in the wild during rain showers. Maintaining naturally high levels
of humidity
also helps to carry the important scents to the nose (Agosta,
1992). Additionally,
enclosures should be reperched every 4-6 months. This gives the
loris a
whole new environment to make its own. In between reperching, the
addition
of fresh floor substrate (see Housing chapter), individual
tree/shrub branches,
and grass clumps intermittently will encourage scent-marking.
.
Socially, lorises are thought to
maintain
individual home ranges, with males’ ranges overlapping the
females’ on
the periphery (Fitch-Snyder, 1995). As with most solitary animals
this
makes scentmarking the cornerstone of communication. In order to
mimic
this situation in captivity, it would be ideal to have several
enclosures
that have access (i.e. through transfer doors) to one common area.
In order
to prevent unwanted copulations or fighting, individuals could
have access
to these common areas singly or with cagemates. For periods of
time, they
could be allowed access alternating with other individuals in
adjoining
cages. These enclosures would allow a natural level of
communication between
a captive population of individuals that, without this set-up,
would most
likely not “interact.” This alone could provide hours of activity.
If this
is not possible, scent-markings of other species that would occur
in that
region or any animal scents including lanolin, African civet musk,
or coendu
porcupine rubbings, just to name a few, would be stimulating. This
can
mimic other species passing through their territory. Feces of
other species
put on the substrate could definitely peak a loris’ interest,
especially
if it could be a potential predator. The introduction of feces and
scent
marks has potential disease risks, so the zoo veterinarian should
first
approve these items. General scents of herbs and spices can also
add interest.
Some favored ones have been fennel, catnip, and freshly picked
mint stalks.
.
The bulk of loris activity periods
in
the wild are allocated to the procurement of food. Unfortunately,
the limit
of enclosure size in captivity greatly reduces the time spent on
this activity.
One of the most basic and straightforward strategies for
enrichment is
to come up with as many ways as possible to make lorises “work for
their
supper.” Presentation of food items tests the creativity of any
keeper.
Lorises collect most of their food as they move in and amongst the
branches.
Placing food items on branches, stabbing them on twigs, smearing
softer
food items on vertical surfaces, and stuffing food into
pre-drilled holes
in perching can greatly increase foraging time. This also gets the
loris
to use all areas of its enclosure in the pursuit of food. In this
scenario,
the smaller the portions of the diet, and the more places it is
hidden,
the more time it takes to find it. An alternative to this that can
be done
intermittently, is to provide a large chunk of fruit or vegetable
either
stabbed onto perching or suspended using a screw with a paperclip
to attach
to mesh or perching. This has a great side benefit for lorises
that are
prone to gingivitis, as they are unable to pop the whole item into
their
mouths and are forced to rub their gums along the surface of a
firm food
item. (Apple and yam are great for those dentally challenged that
no longer
have the ability to bore into wood). Note: of course with any
large amount
of food presented, adjustments must be made to their diet
accordingly.
.
Other forms of food presentation
include
scattering items on the leaf litter substrate and hiding food in
boxes
or sacks. Lorises have tight-gripping prehensile hands with
pseudo-opposable
thumbs, but dexterity is not their forte. For this reason, hiding
food
inside of objects has to be much more straightforward than it
would be
for a simian. Paper sacks filled with hay provide a novel space
that they
have to pick through in order to find insects or other food items.
This
same paper sack with live crickets inside, with the top folded
over, offers
great auditory stimulation. Lorises will circle the bag
84
 Loris
Husbandry Manual
Loris
Husbandry Manual
for quite some time; after a while a small hole can be added at
one
end that allows crickets to slowly exit and gives the loris a
place to
possibly work the bag open. There appears to be quite a bit of
individual
variation in their ability to do this. One loris was intrigued
that something
was there, but he walked away after some time. Another loris
immediately
began to rip up a bag or box with her mouth, using her tight
gripping hands
to brace the box/bag. She began doing this after only a few
introductions
to the set-up. It has been suggested that an enriched environment
may increase
an animal’s general ability to learn (Rosenzweig et. al., 1972).
It may
be possible that even if an animal is doing a task that is not
identical
to one that it would perform in the wild, the fact that it is
doing a task
and learning allows it to maintain a greater capacity for
learning.
.
Vegetation for stimulation
.
Plants are an excellent enrichment
item.
Edible browse provides a loris with bark to strip or bore holes
into, leaves
to eat or give cover, fruits, buds, and flowers to sniff and eat,
or a
new climbing structure to scent-mark, and all this can be provided
with
the addition of one branch (see Table 29, Browse list). The
offerings will
of course vary seasonally and regionally, but variety is best.
Grass clumps
are another easy addition; adding one with dirt intact around the
roots
brings in invertebrates to be consumed or just observed. Lorises
will drag
their bottoms across it to scent-mark. Mealworms or other insects
can be
put on top of the grass and will move down into the grass for
cover, making
it more difficult for the loris to retrieve them. This is another
item
that is easily changed and provides fresh scents.
.
Conclusion
.
The benefits of enrichment are
many. The
most important and beneficial reason for maintaining animals in
captivity
is to educate the public. An enriched animal with a natural set of
activities
will highlight that species’ intrinsic value, and its role in the
larger
ecosystem. Instilling people with the intrinsic value of each
species teaches
respect for animals and their role in the world. By stimulating
lorises
through enrichment, their activity level increases, which piques
the public
interest in these slow-moving primates.
Realistically, zoos will never be
able
to recreate all the variables of a complex, dynamic ecosystem and
the selective
pressures so important to species adaptability and eventual
radiation throughout
a region. The goal then should be to incorporate as many variables
as possible
in the captive habitats and apply new information as it becomes
available.
Most importantly, our knowledge of how ecosystems maintain species
vigor
compared to zoos should be kept in the foremost of all our minds.
Finally,
zoos can be an important educational means to an end. However, the
conservation
of species lies only in the preservation of habitat and most
importantly,
complete ecosystems.
.
Following are instructions for
specific
enrichment programs used at Woodland Park Zoo.
PVC Insect feeder
-
PVC tube (any small diameter; a three inch one works well)
-
caps to fit each end of tube
-
drill with 3/8" drill bit
-
insects
Drill holes all around the tube.
3/8"
seems to be a good size; adult crickets can exit but not quickly.
.
85
 Loris
Husbandry Manual
Loris
Husbandry Manual
This feeder dispenses the insects slowly and randomly, whether the
loris moves this tube or the insects come out on their own. Most
lorises
will watch and/or listen for insect movement and capture once they
exit.
Go Fish
-
shallow pan (e.g., 9"X13" baking pan. Pyrex is good; it allows
lorises
to watch from the side)
-
goldfish (1-2/loris) monitor the amount; some lorises will
readily consume;
others don’t seem to like the taste but will capture and kill.
-
lettuce
Place water and goldfish first.
Once the
loris comprehends fishing, small pieces of romaine can be added
for the
fish to hide under, thereby increasing the challenge for the
loris. This
setup can be placed on the floor of the enclosure. Insects can be
substituted
for goldfish (e.g., giant mealworms and crickets).
.
Table 29: Browse list.
|
Plants
|
Flowers
|
Fruits
|
Exhibit
plants
|
|
Acer
(maple)*
Alnus
(alder)*
Bamboo
(Poadeae)
Betula
(birch)*
Buddleia
(butterfly
bush)
Camelia
Cornus
(dogwood)
Corylus
(hazelnut)
Cotoneaster
Cratageus
(hawthorne)#
Eleagnus#
Escallonia
Fagus
(beech)
Ficus
Foeniculum
vulgare
(fennel)
Hibiscus
Loricera
(honeysuckle)
Mentha
(mint)
Nepeta
(catnip)
Philadelphus
coronarious
Populus
(poplar)**
Rubus
(berries)#
Gaultheria
shallon
(salal)
Salix
(willow)
Spirea
Ulnus
(elm)*
Viburnum
Vitis
(grapes)
|
Hibiscus
(summer)
Rosa
rugosa (summer)
Viburnum
(specifically
high bush cranberry, late spring)
Camelia
(spring)
Loricera
(spring/summer)
Philadelphus
coronarious
(summer)
|
Rubus
(wild
berries, late summer)
Rosa
rugosa (hips,
late summer)
Vitis
(summer)
Viburnum
(specifically
high bush cranberry, late summer / fall)
|
Good
leaf
retention:
Bamboo
Betula
Camelia
Eleagnus
|
** most favored *
favored
# thorns could cause slight injury, use cautiously
Hanging Pine Cones
pine cones (preferably wide open ones)
syringe
gum arabic or honey
nylon string (approximately 12" hang per pine cone)
paper clip
86
Habitat design
Wind the string around the cone several times, and attach the
string
to a paper clip for easy attachment in the enclosure. Thick gum
arabic,
honey or honeycomb can be injected deep into the cone. Make the
honey hard
to reach. Cones can also be used as hiding places for bits of
regular diet.
Treat Log
log (2"-3" diameter, approx. 12" long, soft woods, such as
willow and poplar,
are best)
drill with 3/8" drill bit
plastic tie (use dark colored ties in exhibits)
food item of choice
Cut a section of wood. Drill a hole
at
one end, so that a plastic tie can be threaded through it to
attach to
existing perching. Drill holes randomly all around the log; do not
drill
all the way through. Retain food items and leave spaces for the
loris to
bore into the wood. This encourages a natural woodboring behavior
that
supposedly is a way to obtain carbohydrates and mark their
territory. A
variety of food items can be injected/stuffed into the holes;
e.g., honey,
gum acacia, and bananas. Most lorises will chew into the wood well
beyond
the original hole’s diameter. Any type of edible wood can be used.
.
Table 30: Specialty foods -
Items
beyond the scope of most regular diets. Given randomly throughout
the year
(fruits as they come into season).
| Vegetables |
Fruits |
Live Food |
Misc. Foods |
Squash, pumpkin
(cooked or raw)
Asparagus
Water chestnuts
Beets
Green pepper
Jicama*
Corn on the cob
Zucchini
Cucumber
Green beans*
Celery
Snow and snap peas
Herbs:
Fennel stalks
Chives
Catnip
Oregano
Mint
Dill
Basil
Tarragon
|
Kiwi
Pineapple*
Coconut (whole or pieces)*
Mango* (offer the pit so loris will work to get off
remaining fruit)
Pears
Cherries
Durian fruit* (SE Asia, wild-caughts love, some
captive-born refuse)
Papaya
All berries (let lorises pick off branch if possible)
Dried fruits (use sparingly, esp. with animals that have
existing tooth
problems) |
Feeder
goldfish
Crickets* (various life stages, 2-week olds are good
for lorises in
need of calorie reduction)
Waxworms*
Mealworms* (larvae, pupae, and beetle)
Grasshoppers
|
Gum arabic
Chew-eez rawhide (esp. with barbeque flavoring, some will
chew
and get gum work)
Honeycomb
Sugarcane |
* Favored in the Woodland Park population
87
 Loris
Husbandry Manual
Loris
Husbandry Manual
Bamboo, the handheld treat log
bamboo (1" diameter and 8"-12" sections)
stuffable food item
Cut bamboo into sections (using
loppers
works well). Make it long enough that the loris can’t easily pry
out the
food item from the ends. Ideally, the loris will be encouraged to
try to
bore through the bamboo to get to the hollow center for the food.
Using
choice foods (e.g., dried fruits) is sometimes necessary to keep
them motivated
to do this hard task. The shorter length will allow lorises to
carry the
bamboo log.
.
Snake Shed/Rubber Snake
snake shed (must be gassed with anprolene to kill salmonella
and other
bacteria)
rubber snake
exhibit with lots of places to hide
The snake skin can be frightening
to lorises;
ensure the skin does not remain too long in the exhibit,
particularly if
the animal is overly frightened. Having appropriate covered areas
to retreat
into is very important to allow the loris some control over this
introduced
predator. The rubber snake could be wrapped around a branch or
placed on
the floor. The same can be done with the shed. Response to this
varies.
.
Hammock
cloth (old mealworm bag or burlap)
four plastic ties
hay or leaves
insects or other food items
Make four holes slightly inset, one
at
each corner. Thread the plastic ties through these holes. Suspend
it within
the enclosure to make a quasi-hammock. Initially, the hammock was
to have
been a possible sleep site, but lorises appear to like sleeping on
non-moving
surfaces only. The hammock works well as an area in the branches
in which
to hide food and insects. Moss (Sphagnum-type) also works
well as
an insect hiding spot when placed randomly in the forks of
branches.
.
Bag O’Bugs
paper lunch sack
hay or leaves
live insects (crickets and giant mealworms make the most
noise)
Fill bag half-full with chosen
substrate,
add insects, roll down the top of the bag and place on enclosure
floor.
If the loris listens but does not attempt to break in, make a
small hole
in the bag (large enough for insects to randomly crawl through).
With repeated
exposure to this item, most lorises will get inside.
.
Mixed
Species
Housing
Contributed by Barbara Lester
.
The three main reasons for housing
more
than one species together are conservation/utilization of space,
public
education, and animal enrichment. Each of these issues stand alone
in their
importance, but combined, make a more compelling reason to advance
efforts
in mixing species. Although this is not a new concept, the
documentation
of these efforts is new. Knowing which
.
88
Habitat design
species were housed together and the processes that were involved
is
vital information for animal managers. Information concerning the
three
species of loris housed in polyspecific groups came partly from
the Prosimian
Regional Collection Plan published in 1994 (Porton, 1994) and by a
survey
done in December 1996 of 23 U.S. facilities either holding lorises
( 19
zoos/1 university) or having held them historically (3 zoos).
.
In 23 North American institutions,
the
survey found that 43 % had mixed species with one or more loris
species.
The only polyspecific primates that were housed with lorises were
nocturnal
prosimians (8 species). The other polyspecifics, (12 species),
involved
in mixed species exhibits were nocturnal mammals, with two
exceptions:
tree shrews, a diurnal mammal, were housed with slender lorises.
The other
exception was the only overtly unsuccessful mixed species
situation reported.
A Tokay gecko (reptile) was mixed with a loris species and was
unfortunately
eaten by the loris. All other institutions reported overall
success with
housing lorises with polyspecifics.
.
The only problems found were
obesity in
some of the lorises or the other prosimians. The husbandry issue
of how
to feed each species independently of the other became problematic
in some
situations. The lack of control over the food consumption and the
type
of food required for each species were pointed out as factors to
be considered
when mixing lorises with polyspecifics. As an example, slow
lorises housed
with giant fruit bats, Egyptian fruit bats and aardvarks presented
a feeding
problem in one facility. The soft produce fed to the bats was
eaten by
the lorises and more feeding stations were needed for the bats
where the
lorises could not avail themselves of the food. In addition, a
potential
dental problem was reported in this situation because of too much
soft
produce being eaten by the lorises with no way to limit their
access in
this particular exhibit. Although these as well as many other
potential
problems are faced when mixing any species, animal managers found
these
as the only major problems to be considered when planning a mixed
species
exhibit which includes lorises.
.
Editor’s Note: After the
section
on Mixed Species Housing was written, we received a report of
three slow
loris deaths at Cleveland Zoo due to Pasteurella. The lorises
apparently
contracted this bacteria from a Prevost’s squirrel (Callosciurus
prevostii)
that was housed in the same enclosure and showed no signs of
illness. Although
highly desirable from a management point of view, mixed species
housing
can also carry potential disease risks that should be considered.
89
 Loris
Husbandry Manual
Loris
Husbandry Manual
Table 31: Species and the number of individuals that have
been successfully kept in same exhibits as pygmy lorises.
Regarding number
of individuals, the first number refers to males, the second
number females,
and if a third number is given the gender was unknown. The data is
based
on survey in December 1996.
| Pygmy
Loris Nycticebus
pygmaeus |
| Institution |
# of
Individuals |
Other Species |
Enclosure
Size |
| Duke
University Primate
Center |
1.1 |
1.1 Slender
Loris
1.0 Bushbaby
1.1 Coquerel's Mouse Lemur |
4.5m x 2.7m x
2.7m |
| 1.1 |
1.0 Bushbaby
1.1 Coquerel's Mouse Lemur |
4.5m x 4.5m
x 2.7m |
| 1.1
NOTE: 0.1 became pregnant, removed for birth,
reintroduced with juvenile
Reconfigured group 3.1
|
1.2 Slender
Loris
(0.1 juvenile)
1.1 Potto
2.0 Bushbaby
1.1 Slender Loris
1.1 Potto
1.1 Bushbaby
|
4.5m x 2.7m
x 2.7m |
| Brookfield Zoo |
1.3 (0.1
juvenile, 0.1 infant)
NOTE: Reconfigured group; 0.1 (older juvenile)
+ 0.1 (juvenile) introduced to group later
|
0.1 Slender
Loris
1.1 Slender Loris
(1.0 introduced after the females were together)
|
1.8m x 1.2m x 2m
(Initial introduction
done in two cages of this size.) |
90
Table 32: Species and the
number
of individuals that have been sucessfully kept in same exhibits as
slow
lorises.
| Slow
Loris Nycticebus
coucang |
| Institution |
# of
Individuals |
Other Species |
Enclosure
Size |
| Duke
University Primate
Center |
0.2
|
1.0 Aye Aye
(slept side-by-side
with loris)
1.1 Bushbaby |
4.5m x 6m x 2.7m |
|
0.2
|
1.0 Aye Aye
1.1 Potto
1.1 Bushbaby |
4.5m x 6m x
2.7m |
|
1.1
|
1.0 Aye Aye
1.0 Slender Loris
1.2 Bushbaby
0.2 Coquerel's Mouse Lemur |
82m3
hexagonal room |
|
1.1
|
1.0 Aye Aye
1.1 Slender Loris
0.2 Coquerel's Mouse Lemur
1.1 Potto |
4.5m x 6m x
2.7m |
|
1.1
|
2.0 Bushbaby
0.2 Coquerel's Mouse Lemur
1.1 Potto
1.1 Lesser Mouse Lemur (removed due to obesity) |
4.5m x 6m x
2.7 m |
| Cincinnati
Zoo |
1.1 (bred and
reared young) |
1.2 Malayan
Mouse Deer |
1.8m x 1.2m
x 1.8m |
|
1.1
|
0.0.1 Echidna |
unknown |
|
1.1
|
0.0.1 Aardvark
Giant Fruit Bat Colony
Egyptian Fruit Bat Colony |
3.6m x 3m x
2.4m |
|
1.1
|
1.0 Betton |
unknown |
| Bronx
Zoo/Wildlife Conservation
Park |
1.1
|
1.1 Malayan
Mouse Deer |
1m x 2m x 1.5m |
| Audubon Park and
Zoo |
1.1 geriatric |
2.0 Bushbaby |
3m x 3m x 2.4m
indoors, glass
fronted |
| Minnesota
Zoo |
l. l |
0.1 Asian
Crested Porcupine |
6m x 4.5m x 6m |
| 0.1 |
0.2 Tree Shrews |
1.8m x 1.8m
x 3.6m glass fronted |
| Santa Barbara
Zoo |
1.1 |
1.0 Sugarglider |
2.1m x 2.4m x
1.5m |
91
 Loris
Husbandry Manual
Loris
Husbandry Manual
Table 33: Species and the
number
of individuals that have been sucessfully kept in same exhibits as
slender
lorises.
| Slender
Loris
Loris
tardigradus |
| Institution |
# of
Individuals |
Other Species |
Enclosure
Size |
| Duke
University Primate
Center |
1.2 (0.1
juvenile) |
1.1 Potto
3.1 Pygmy Slow Loris (removed due to obesity and
pregnancy)
1.1 Bushbaby
0.3 Fat-Tailed Dwarf Lemur |
4.5m x 2.7m x
2.7m indoor enclosure |
| 1.1 |
2.0 Tarsier
1.1 Pygmy Slow Loris |
4.5m x 2.7m
x 2.7m indoor enclosure |
| 1.1 (removed
after one year due to obesity) |
1.1 Tarsier |
unknown |
| 1.1 |
1.0 Bushbaby
1.1 Coquerel's Mouse Lemur
1.1 Pygmy Slow Loris
0.2 Fat-Tailed Dwarf Lemur (removed due to obesity) |
4.5m x 2.7m
x 2.7m indoor enclosure |
| 1.1 |
1.0 Aye Aye
1.1 Pygmy Slow Loris
0.2 Coquerel's Mouse Lemur
1.2 Galago
1.1 Potto |
4.5m x 6m x
2.7 indoor enclosure |
| 1.1 |
0.2 Fat-Tailed
Dwarf Lemur
0.2 Bushbaby
0.2 Coquerel's Mouse Lemur |
4.5m x 2.7m
x 2.7m indoor enclosure |
| Cincinnati Zoo |
1.1 (bred and
reared young) |
1.2 Malayan
Mouse Deer |
1.8m x 1.2m x 1.8m
|
| Philadelphia
Zoo |
0.1 - |
1.3 Tree Shrews |
2.7m x 0.76m x
1.6m |
| 1 |
1.3 Tree Shrews |
same as above |
| 0.1 |
Longeared Hedgehogs |
same as above |
| 1 |
Longeared Hedgehogs |
same as above |
| 1 |
0.0.1 Greater
Hedgehog Tenrec |
same as above |
| 0.1 |
0.0.1 Greater
Hedgehog Tenrec |
same as above |

92
Privacy policy / Datenschutz
Back to the top of this page
|
Management
of Lorises in Captivity. A Husbandry Manual for
Asian Lorisines (Nycticebus
& Loris ssp.)
Edited
by: Helena
Fitch-Snyder and Helga Schulze. Compiler: Lena C.
Larsen
|
Last
amendment: 2 January 2003
|
![]() Loris
Husbandry Manual
Loris
Husbandry Manual



![]() Loris
Husbandry Manual
Loris
Husbandry Manual
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
