| Home |
|
|
Husbandry Manual for Asian
Lorisines
(Nycticebus & Loris ssp.)
| HEALTH |
|
| Review of Loris
Clinical Information
and Pathology Data from the San Diego Zoo: 1982-1995
-contributed by Meg Sutherland Smith, D.V.M. and Ilse Stalis, D.V.M. . |
|
| Introduction |
|
| Preventive Medicine |
|
| Anesthesia |
|
| Clinical Pathology |
|
| Medical Review |
|
| Summary |
|
| Tables |
|
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
HEALTH
Review Of Loris Clinical Information And Pathology Data From The San Diego Zoo: 1982-1995
Contributed by Meg Sutherland-Smith, D.V.M., and Ilse Stalis, D.V.M.
There is very little published information regarding the medical management of lorises. The following is a review of clinical and pathology data from the San Diego Zoo since 1982 for three species of loris: Loris tardigradus, Nycticebus pygmaeus, and Nycticebus coucang. A summary of mortalities seen at the Duke University Primate Center (Tables 22-24), as well as some clinical data is also included. It is by no means complete but is a start in the compilation of medical information for loris species. Additional and new health information will be published via the Internet in the species.net health database.
Each individual receives an annual examination, including tuberculin testing (upper eyelid using mammalian old tuberculin, 0.1 cc intradermal), complete blood count (CBC), chemistry panel, and rectal culture. Loris rectal temperatures are lower than those expected for other mammalian species; rectal temperatures of anesthetized animals ranges from 86-94½ F (30-30.4½ C) for slender lorises, 88-98½ F (31-36.6½ C) for pygmy lorises, and 92-98½ F (33.3-36.6½ C) for slow lorises. Whittow et al. (1977) reported mean rectal temperatures of unanesthetized lorises as 95.5½ F (35.3½ C) and 96.3½ F (35.7½ C) for day and night measurements respectively. Both Whittow et al. (1977) and Müller (1975) have reported oxygen consumption rates lower for slow lorises than would be expected for a placental mammal and have described the slow loris as a hypometabolic primate. Blood samples can be obtained from either the jugular or femoral vein. The jugular vein is rarely visible or palpable; localization of the jugular vein is based on approximation and pulsations from the carotid artery. Venipuncture is a challenge in slender lorises due to their small size. Animals are vaccinated for tetanus toxoid approximately every five years. Routine fecal exams are performed biannually. Routine weighing is also an important part of the preventive health program. Weight loss trends may not be evident by visual examination and are frequently an indication of an underlying disease process. If not monitored closely, the slow and pygmy lorises have a tendency to become obese.
Because of their size many lorises can be manually restrained for short, simple procedures.
Health
| Anesthetic agent | Ketaminea | Tiletamine/Zolazapamb | Isofluranec |
| Loris tardigradus | NDd | ND | Yes |
| Nycticebus pygmaeus | ND | 5-28 mg/kge | Yes |
| Nycticebus coucang | 10-25 mg/kg | 4014 mg/kg | Yes |
a Ketaset, Fort Dodge Laboratories Inc., Fort Dodge, Iowa, 50501 USA.
b Telazol, Fort Dodge Laboratories Inc., Fort Dodge, Iowa, 50501 USA. Provides better relaxation than ketamine.
c Aerrane, Ohmeda PPD Inc., Liberty Corner, New Jersey, 07938 USA.
d ND- use not documented in the SDZ collection.
e A wide range of dosages have been used but 8-12 mg/kg is the average dose used. Slow recoveries are associated with the higher doses.
Clinical pathology data is listed at the end of this section. This information has been provided by MedARKS, International Species Information System (ISIS). Unfortunately several large institutions housing loris colonies are not currently part of this data base.
Medical records and necropsy information since 1982 for each animal were reviewed. Clinical problems were classified based on organ system affected or general disease category.
Table 21: Population
demographics
for loris species reviewed.
| Loris tardigradus | Nycticebus pygmaeus | Nycticebus coucang | |
| Animals accessioned | 3.5 | 6.8.2 | 15.14 |
| Births | 0.2 | 3.4.2 | 5.3 |
| Deaths: neonate | 0 | 1.1 | 0 |
| Deaths: juvenile | 0 | 0.1 | 0 |
| Deaths: adult | 0.1 | 2.1 | 7.7 |
Cardio-pulmonary disease
Pygmy loris: One animal was diagnosed antemortem with pneumonia based on radiographic evaluation. Enterobacter sp. and Proteus sp. were cultured from a tracheal wash.
Slow loris: A pronounced heart murmur was noted in one animal. Myocardial amyloidosis and fibrosis were noted histologically at necropsy. One animal was treated several times for sneezing,
61
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
congestion, and a purulent nasal discharge. This animal had severe dental disease; the upper respiratory infection may have been secondary to oronasal fistulas. Acute pneumonia was seen in two slow lorises at necropsy.
Renal disease
Pygmy loris: Evidence of renal impairment based on clinical chemistries was seen in one animal. Chronic interstitial nephritis was seen in this as well as one other individual at necropsy.
Slow loris: Four slow lorises had indications of impaired renal function. Clinical chemistries revealed E. coli was subsequently cultured from the urine of one of these animals. Another loris with a consistently urine soaked gluteal area was noted to be polydipsic and polyuric. This loris experienced episodes of azotemia and inappetence. Chronic interstitial nephritis was diagnosed histologically at necropsy. Varying degrees of chronic interstitial nephritis were noted in six animals at necropsy. Calcification of the aorta and periosteal proliferation of the proximal ulnas were seen in one loris euthanized due to renal failure.
Hepatic disease
Slender loris: At necropsy, a gall stone was found in a three year old male slender loris housed at Cincinnati Zoo. No other liver pathology was grossly evident. This slender loris had a history of neuromuscular abnormalities (Schulze, personal communication).
Pygmy loris: One loris had an elevated ammonia on serum chemistries. Bile acids were also elevated. Cholelithiasis was seen in this individual, as well as one other, at necropsy.
Neurologic and musculoskeletal disease
Slender loris: One animal was treated for a stifle abscess that healed without complications. Staphylococcus epidermidis was cultured from the abscess. It is not known if this was secondary to a bite wound or other trauma.
Pygmy loris: An adult female in late term gestation developed sudden-onset seizures. No cause for the seizures was found antemortem. A thrombosis in the basal artery of the brain was found at necropsy. One animal exhibiting a head tilt was diagnosed with otitis externa/media; this responded to treatment. One loris developed posterior paresis prior to parturition, which resolved post-partum. One animal has been examined twice for episodes of ataxia and tremoring. Nothing definitive has been found on exam and the significance is not clear.
Slow loris: Degenerative changes in the lumbar vertebrae were seen radiographically in a loris that developed posterior paresis. Chondroid degeneration of the vertebrae was noted histologically at necropsy.
Integumentary system
Pygmy loris: One animal was examined for a focal area of skin slough; the etiology was undetermined. Another loris was treated for an ulcerative dermatitis involving both tarsi. Two
62
Dental disease
Pygmy loris: Two animals have been treated for dental disease. One animal had four episodes of facial abscessation associated with dental disease.
Slow loris: Twelve lorises have undergone treatment for dental disease. Five of these have undergone multiple exams for recurrent periodontal disease and/or abscessed teeth. One loris
63
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
developed osteomyelitis of the zygomatic arch. Facial swellings have been a common sign in lorises with dental disease. One loris was treated multiple times over a three year period for purulent ocular discharge; this was eventually resolved (several types of bacteria were cultured on different occasions). This animal also had a history of dental disease. One loris was treated for a retrobulbar abscess involving the right eye. This animal also had a history of dental disease. These conditions were thought to be secondary to dental disease.
Trauma
Slender loris: One slender loris is presumed to have died following a fall from the top of the enclosure. A subdural hematoma was the only gross necropsy finding noted.
Pygmy loris: There have been six trauma events requiring medical attention. These have generally been bite wounds in which cellulitis and abscessation developed. One juvenile loris died secondary to septicemia from bite wounds.
Slow loris: There were eleven episodes of bite wounds which required medical attention. One loris developed septicemia secondary to the bite wounds and died. Several animals have required amputation of one or more digits due to bite wounds. Most bite wounds occurred either during introductions or when males escaped from enclosures and fought with other males through cage wire. Another loris died following complications after having become entrapped in a cargo net used for climbing.
Parasitism
Slender loris: Nematodes (unspeciated) were diagnosed from fecal exams in two animals. Microfilaria identified as Dipetalonema sp. were seen in the tissues of a slender loris at necropsy prior to 1983 (Griner). They were not thought to have any clinical significance.
Pygmy loris: Trichuris was diagnosed on fecal exam in six animals. Giardia has been diagnosed on fecal exam in three animals. Enterobius was noted on fecal exam of one animal. Hymenlopis-like ova were noted on fecal exam in one animal. Microfilaremia in blood samples was noted in three wild caught animals upon arrival. The animals were treated with ivermectin and microfilaremia was not present on subsequent blood samples. Unidentified microfilaria were seen at necropsy in pygmy loris at Duke University Primate Center.
Slow loris: Pterygodermatides
nycticebi
was diagnosed by fecal exam in three animals. A fourth loris
died from anemia secondary to blood loss from gastric parasitism
by Pterygodermatides.
Nonidentified nematodes were diagnosed by fecal exam in four
animals. Tapeworms
were noted on fecal exam of three lorises; each loris was infected
on two
separate occasions.
Giardia sp. was diagnosed by fecal exam in three
lorises. Trichomonas
sp. was seen in the feces of two lorises. One
of these had bloody stool and diarrhea. It is not known whether
the trichomonads
were the cause of the bloody stool, but the condition resolved
with treatment.
Microfilaria in the blood was noted in one wild caught loris. Strongyloides
sp.,
Physaloptera
sp.,
Cryptosporidia
and
oxyurids have also been seen in the slow loris collection at the
Duke University
Primate Center. One animal reportedly died from
Health
Trauma has been a major cause of morbidity and mortality at San Diego Zoo as well as at the Duke University Primate Center. Bite wounds have resulted in the deaths of animals at both institutions. Marked cellulitis with secondary necrosis is a frequent sequelae to bite wounds. Asian folklore depicts the slow loris as a venomous animal (Wilde, 1972). Research has been unable to definitively prove this theory. There is speculation that brachial gland excretions mixed with saliva are potentially toxic, and this could explain the tissue reactions following bite wounds. Animals should be observed closely for evidence of wounds, especially when new introductions are taking place. Any suspected wounds should be followed up with a minimum of a good visual inspection to determine if an examination with anesthesia is necessary. Housing design should not allow physical contact between adjacent enclosures.
Dental disease also contributed significantly to case morbidity. Viral infection has been suggested as a cause for the widespread periodontal disease, but dietary considerations should not be overlooked. Chronic periodontal disease and open root canals can be a source for disseminated bacterial infections. Diets consisting primarily of soft food items should be avoided; some type of biscuit or pellet should be included to promote hygiene. Proper dietary management will reduce the incidence of dental disease. To detect dental problems early a thorough oral evaluation and dental prophylaxis should be performed each time a loris is examined.
Respiratory disease was not as common as trauma and dental disease in this collection; however, upper respiratory infections have been a common clinical entity seen at the Duke University Primate Center. Ten animals have been treated one or more times for an upper respiratory tract infection. Two other conditions frequently seen in the slow lorises at the Duke University Primate Center were
65
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
Meibomian gland abscessation and conjunctivitis.
Renal disease was an important cause of morbidity and mortality, especially in older animals. In a review of Zoological Society of San Diego necropsy data from 1964 to 1978, Griner diagnosed chronic interstitial nephritis in a slender loris and a slow loris. Renal disease contributed to 20% of deaths in slow lorises at the Duke University Primate Center. Renal function should always be evaluated, particularly when examining older animals.
While parasitism was the direct cause of death in only two animals, a variety of parasites have been diagnosed on fecal exam or incidentally at necropsy. Any wild caught animal should be considered parasitized and treated appropriately. There are several papers regarding parasitism in loris species that have not been reviewed but are included in the literature citations.
Table 22: Mortality in Nycticebus
coucang
at the Duke University Primate Center 1980-1994.
| Organ system affected/disease process | Number of animals affected/total |
| Renal disease | 5/24 |
| Pulmonary disease | 4/24 |
| Neonatal mortality | 4/24 |
| Bacterial septicemia | 3/24 |
| Neoplasia | 2/24 |
| Chronic heart failure | 2/24 |
| Chronic sinusitis | 1/24 |
| Parasitic infestation | 1/24 |
| Bite wounds | 1/24 |
Table 23: Mortality in Nycticebus
pygmaeus
at the Duke University Primate Center 1988-1994.
| Organ system affected/disease process | Number of animals affected/total |
| Renal disease | 1/9 |
| Urethral calculi | 1/9 |
| Neoplasia | 1/9 |
| Peritonitis | 1/9 |
| Bite wounds | 1/9 |
| Hemorrhagic syndrome | 2/9 |
| Neonatal mortality | 2/9 |
Table 24: Mortality in Loris
tardigradus
at
the Duke University Primate Center 1985-1994.
| Cause of death | Number of animals affected/total |
| Neonatal mortality | 12/14 |
| Bite wounds | 2/14 |
66
| Test | Mean |
|
N |
|
|
|
|
| Alanine Aminotransferase | 96 | 93 | 29 | 25 | 539 | 23 | 6 |
| Albumin (Colorimetry) | 3.5 | 0.4 | 14 | 2.9 | 4.2 | 11 | 5 |
| Alkaline Phosphatase | 196 | 118 | 29 | 81 | 518 | 23 | 7 |
| Amylase | 1573 | 263 | 5 | 1204 | 1884 | 5 | 2 |
| Aspartate Aminotransferase | 116 | 36 | 19 | 83 | 226 | 16 | 6 |
| Bicarbonate | 22.3 | 4.9 | 3 | 19.0 | 28.0 | 3 | 2 |
| Blood Urea Nitrogen | 16 | 8 | 31 | 5 | 34 | 24 | 8 |
| Body Temperature | 35.5 | 0.9 | 24 | 34.0 | 37.0 | 17 | 4 |
| Calcium | 10.1 | 1.3 | 28 | 7.7 | 13.8 | 22 | 6 |
| Carbon Dioxide | 20.6 | 3.8 | 10 | 16.0 | 28.0 | 7 | 4 |
| Chloride | 109 | 7 | 24 | 98 | 124 | 19 | 7 |
| Cholesterol | 417 | 245 | 22 | 0 | 810 | 17 | 6 |
| Creatine Phoshokinase | 42 | 34 | 6 | 17 | 108 | 6 | 13 |
| Creatinine | 0.7 | 0.5 | 29 | 0.1 | 2.5 | 22 | 6 |
| Eosinophils |
|
|
5 | 0.000 | 0.099 | 5 | 3 |
| Gamma Glutamyltransferase | 25 | 7 | 7 | 10 | 30 | 7 | 2 |
| Globulin (Colorimetry) | 4.1 | 0.8 | 13 | 2.3 | 5.2 | 10 | 5 |
| Glucose | 121 | 40 | 34 | 54 | 245 | 25 | 8 |
| Hematocrit | 42.0 | 5.6 | 54 | 25.0 | 53.0 | 35 | 8 |
| Hemoglobin | 13.9 | 2.0 | 40 | 9.2 | 17.5 | 30 | 7 |
| Iron | 190 | 25 | 4 | 161 | 216 | 4 | 2 |
| Lactate Dehydrogenase | 304 | 288 | 11 | 117 | 1092 | 11 | 5 |
| Lipase | 42 | 0 | 1 | 42 | 42 | 1 | 1 |
| Lymphocytes |
|
|
12 | 1.120 |
|
10 | 5 |
| MCHC | 33.0 | 2.9 | 39 | 26.0 | 38.9 | 30 | 7 |
| Monocytes |
|
|
10 | 0.000 | 0.096 | 9 | 5 |
|
|
24.6 | 2.2 | 18 | 20.6 | 28.1 | 14 | 6 |
| Mean Corpuscular Volume | 72.6 | 4.1 | 18 | 65.1 | 77.9 | 14 | 6 |
| Neutrophilic Bands |
|
|
2 | 0.050 | 0.250 | 2 | 2 |
| Nucleated Red Blood Cells | 2 | 4 | 10 | 0 | 13 | 9 | 6 |
| Phosphorus | 4.1 | 1.9 | 26 | 1.4 | 10.4 | 20 | 6 |
| Platelet Count | 297 | 151 | 7 | 107 | 584 | 4 | 2 |
| Potassium | 4.0 | 0.9 | 26 | 2.5 | 5.8 | 21 | 7 |
| Red Blood Cell Count | 5.84 | 0.70 | 18 | 3.93 | 6.94 | 14 | 6 |
| Reticulocytes | 0.7 | 0.0 | 1 | 0.7 | 0.7 | 1 | 1 |
| Segmented Neutrophils |
|
|
12 | 0.734 | 9.150 | 10 | 5 |
| Sodium | 148 | 8 | 26 | 138 | 168 | 21 | 7 |
| Total Bilirubin | 0.2 | 0.2 | 21 | 0.0 | 0.8 | 17 | 6 |
| Total Protein (Colorime ) | 7.2 | 1.1 | 23 | 4.8 | 8.9 | 19 | 6 |
| Total Protein (Refractometer) | 10.0 | 0.3 | 2 | 9.8 | 10.2 | 2 | 1 |
| Triglyceride | 441 | 535 | 10 | 90 | 1631 | 10 | 3 |
| Uric Acid | 2.1 | 1.0 | 14 | 0.8 | 3.8 | 14 | 4 |
| White Blood Cell Count |
|
|
13 | 3.190 | 17.6 | 11 | 5 |
67
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
Table 26: ISIS Clinical
Pathology
Reference Ranges in Pygmy Loris Nycticebus pygmaeus
as per July
1, 1996.
| Test | Mean | Std.
Dev. |
N | Min.
Value |
Max.Value | # of
Ind. |
# of
zoos |
| Alanine Aminotransferase | 89 | 46 | 15 | 45 | 207 | 11 |
2
|
| Albumin (Colorimetry) | 3.6 | 0.8 | 5 | 2.4 | 4.6 | 5 |
1
|
| Alkaline Phosphatase | 88 | 32 | 9 | 53 | 135 | 7 |
2
|
| Amylase | 570 | 40 | 2 | 542 | 598 | 2 |
1
|
| Aspartate Aminotransferase | 101 | 28 | 14 | 48 | 148 | 11 |
2
|
| Blood Urea Nitrogen | 25 | 10 | 17 | 14 | 45 | 12 |
3
|
| Body Temperature | 35.5 | 1.0 | 11 | 34.0 | 38.0 | 6 |
1
|
| Calcium | 10.2 | 1.3 | 10 | 7.8 | 11.8 | 8 |
2
|
| Carbon Dioxide | 22.0 | 1.0 | 3 | 21.0 | 23.0 | 3 |
1
|
| Chloride | 105 | 4 | 4 | 101 | 109 | 4 |
1
|
| Cholesterol | 302 | 41 | 10 | 233 | 379 | 8 |
2
|
| Creatine Phophokinase | 138 | 149 | 4 | 43 | 360 | 4 |
1
|
| Creatinine | 0.3 | 0.1 | 5 | 0.3 | 0.4 | 5 |
3
|
| Gamma Glutamyltransferase | 47 | 25 | 9 | 19 | 91 | 7 |
2
|
| Globulin (Colorimetry) | 3.0 | 0.3 | 5 | 2.6 | 3.4 | 5 |
1
|
| Glucose | 181 | 80 | 15 | 102 | 422 | 11 |
2
|
| Hematocrit | 42.1 | 6.7 | 21 | 24.9 | 51.0 | 14 |
4
|
| Hemoglobin | 15.6 | 3.2 | 20 | 8.6 | 23.0 |
4
|
|
| Lactate Dehydrogenase | 137 | 36 | 4 | 95 | 180 | 114 4 |
1
|
| Magnesium | 3.10 | 0.57 | 2 | 2.70 | 3.50 | 2 |
1
|
| MCHC | 37.3 | 7.8 | 20 | 20.0 | 63.9 | 13 |
4
|
|
|
26.0 | 3.3 | 20 | 16.6 | 31.4 | 13 |
4
|
| Mean Corpuscular Volume | 71.6 | 21.9 | 21 | 43.2 | 154.1 |
4
|
|
| Nucleated Red Blood Cells | 1 | 0 | 6 | 1 | 2 | 6 |
2
|
| Phosphorus | 6.1 | 2.1 | 4 | 4.2 | 8.9 | 4 |
1
|
| Platelet Count | 440 | 235 | 5 | 134 | 781 | 5 |
1
|
| Potassium | 3.9 | 0.6 | 4 | 3.0 | 4.4 | 4 |
1
|
| Red Blood Cell Count | 6.21 | 1.66 | 21 | 3.31 |
|
14 |
4
|
| Sodium | 143 | 1 | 4 | 142 | 144 | 4 |
1
|
| Total Bilirubin | 0.3 | 0.2 | 9 | 0.1 | 0.6 | 7 |
1
|
| Total Protein (Colorimetry) | 6.5 | 0.8 |
|
4.8 | 7.6 | 11 |
2
|
| Triglyceride | 79 | 35 |
|
19 | 150 | 8 |
2
|
| Uric Acid | 1.5 | 0.6 | 6 | 0.9 | 2.3 | 5 |
2
|
68
| Test | Mean | Std.
Dev. |
N | Min.
Value |
Max.
|
# of
|
# of
|
| Alanine Aminotransferase | 64 | 13 | 4 | 48 | 79 | 4 | 1 |
| Albumin (Colorimetry) | 4.1 | 0.0 | 1 | 4.1 | 4.1 | 1 | 1 |
| Aspartate Aminotransferase | 47 | 18 | 5 | 33 | 76 | 5 | 1 |
| Blood Urea Nitrogen | 39 | 7 | 7 | 27 | 47 | 6 | 2 |
| Calcium | 10.0 | 0.0 | 1 | 10.0 | 10.0 | 1 | 1 |
| Carbon Dioxide | 24.0 | 0.0 | 1 | 24.0 | 24.0 | 1 | 1 |
| Chloride | 113 | 2 | 2 | 111 | 114 | 2 | 1 |
| Cholesterol | 228 | 18 | 2 | 215 | 241 | 2 | 1 |
| Creatinine | 0.3 | 0.1 | 2 | 0.3 | 0.4 | 2 | 2 |
| Eosinophils | 0.001 |
|
1 | 0.001 | 0.001 | 1 | 1 |
| Globulin (Colorimetry) | 2.4 | 0.0 | 1 | 2.4 | 2.4 | 1 | 1 |
| Glucose | 182 | 60 | 7 | 103 | 264 | 6 | 1 |
| Hematocrit | 43.3 | 5.9 | 9 | 30.0 | 50.2 | 8 | 3 |
| Hemoglobin | 15.1 | 1.6 | 9 | 11.6 | 16.9 | 8 | 3 |
| Lymphocytes | 7.040 |
|
1 | 7.040 | 7.040 | 1 | 1 |
| MCHC | 35.1 | 1.8 | 9 | 32.5 | 38.7 | 8 | 3 |
| Monocytes | 0.000 |
|
1 | 0.000 | 0.000 | 1 | 1 |
| Mean Corpuscular Hemoglobin | 27.7 | 1.6 | 9 | 23.9 | 29.4 | 8 | 3 |
| Mean Corpuscular Volume | 78.9 | 4.1 | 9 | 71.4 | 83.5 | 8 | 3 |
| Nucleated Red Blood Cells | 11 | 17 | 3 | 0 | 31 | 3 | 2 |
| Phosphorus | 3.9 | 0.0 | 1 | 3.9 | 3.9 | 1 | 1 |
| Platelet Count | 150 | 0 | 1 | 150 | 150 | 1 | 1 |
| Potassium | 4.0 | 0.0 | 1 | 4.0 | 4.0 | 1 | 1 |
| Red Blood Cell Count | 5.47 | 0.63 | 9 | 4.20 | 6.37 | 8 | 3 |
| Segmented Neutro hits | 0.980 |
|
1 | 0.980 | 0.980 | 1 | 1 |
| Sodium | 148 | 1 | 2 | 147 | 148 | 2 | 1 |
| Total Bilirubin | 0.3 | 0.1 | 6 | 0.2 | 0.5 | 5 | 1 |
| Total Protein (Colorimetry) | 6.1 | 0.6 | 6 | 5.5 | 7.0 | 5 | 1 |
| Uric Acid | 1.8 | 0.5 | 2 | 1.4 | 2.1 | 2 | 1 |
| White Blood Cell Count | 8.910 |
|
1 | 8.910 |
|
1 | 1 |
69
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
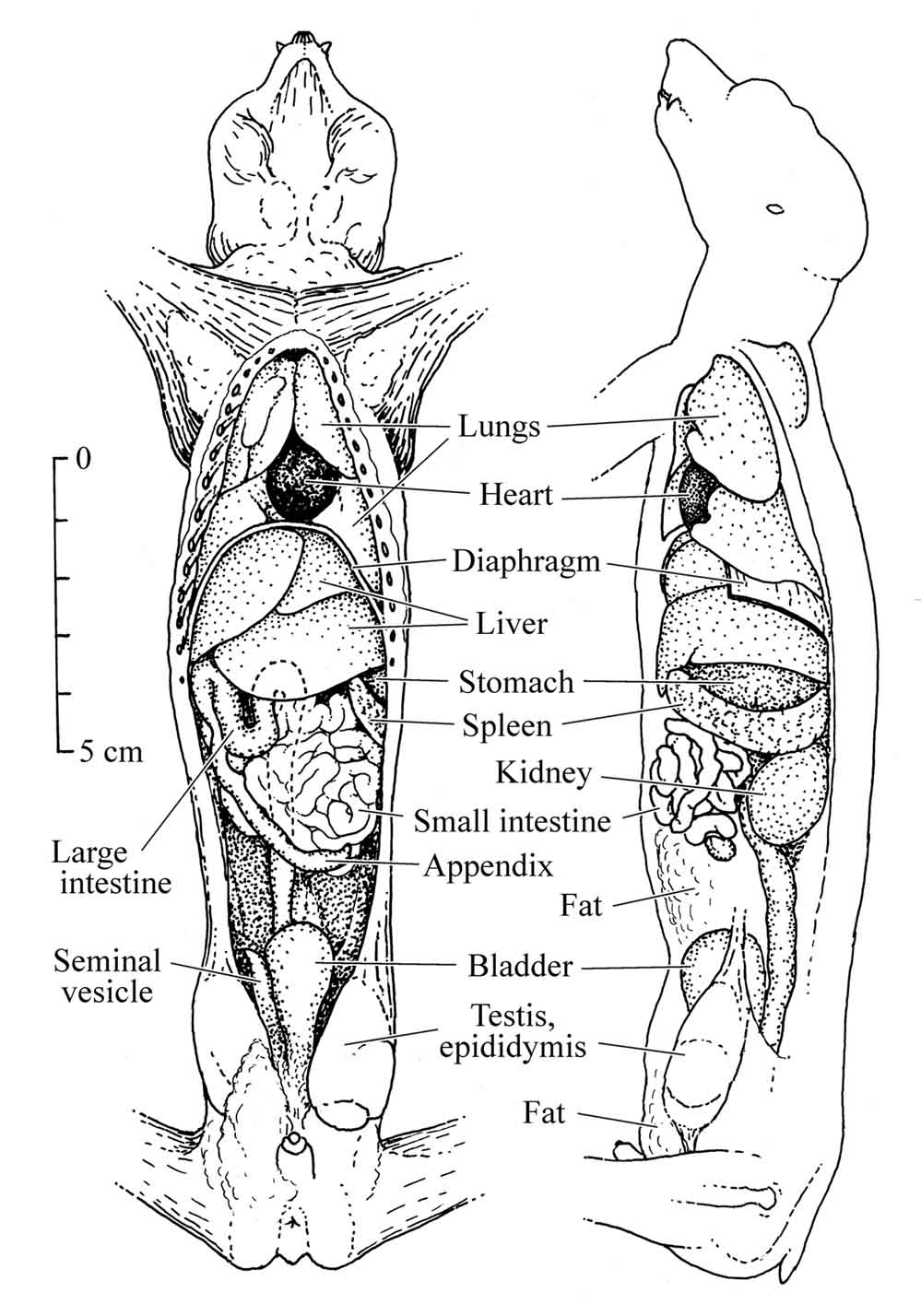
Figure 26: Anatomy of the slender loris |
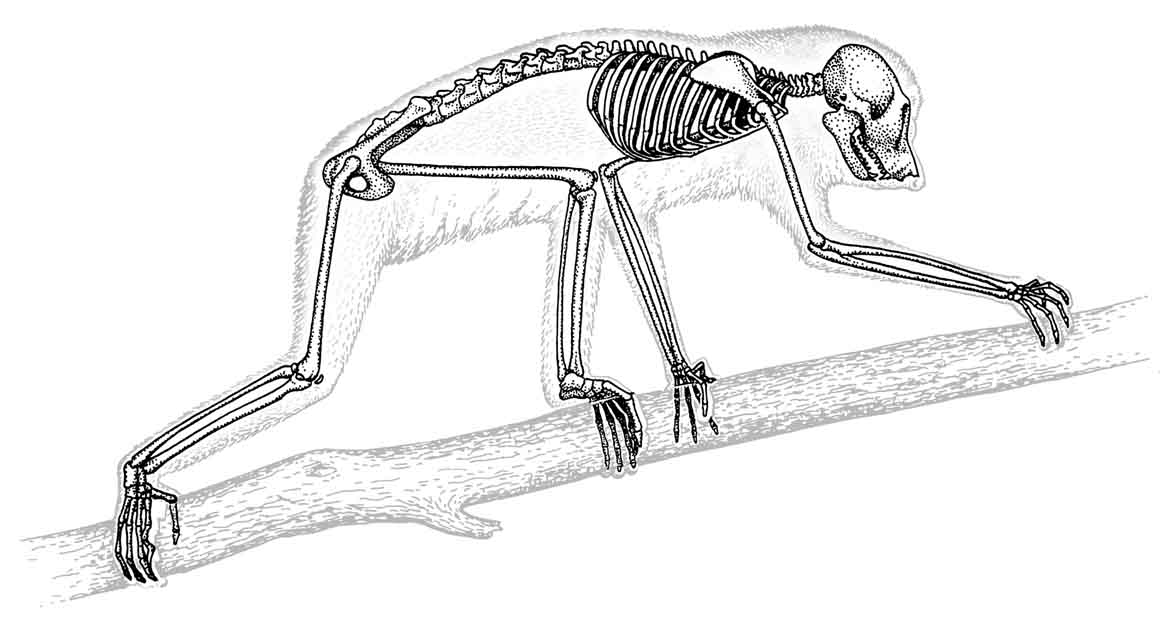
Figure 27: Skeletal structure of the slender loris |
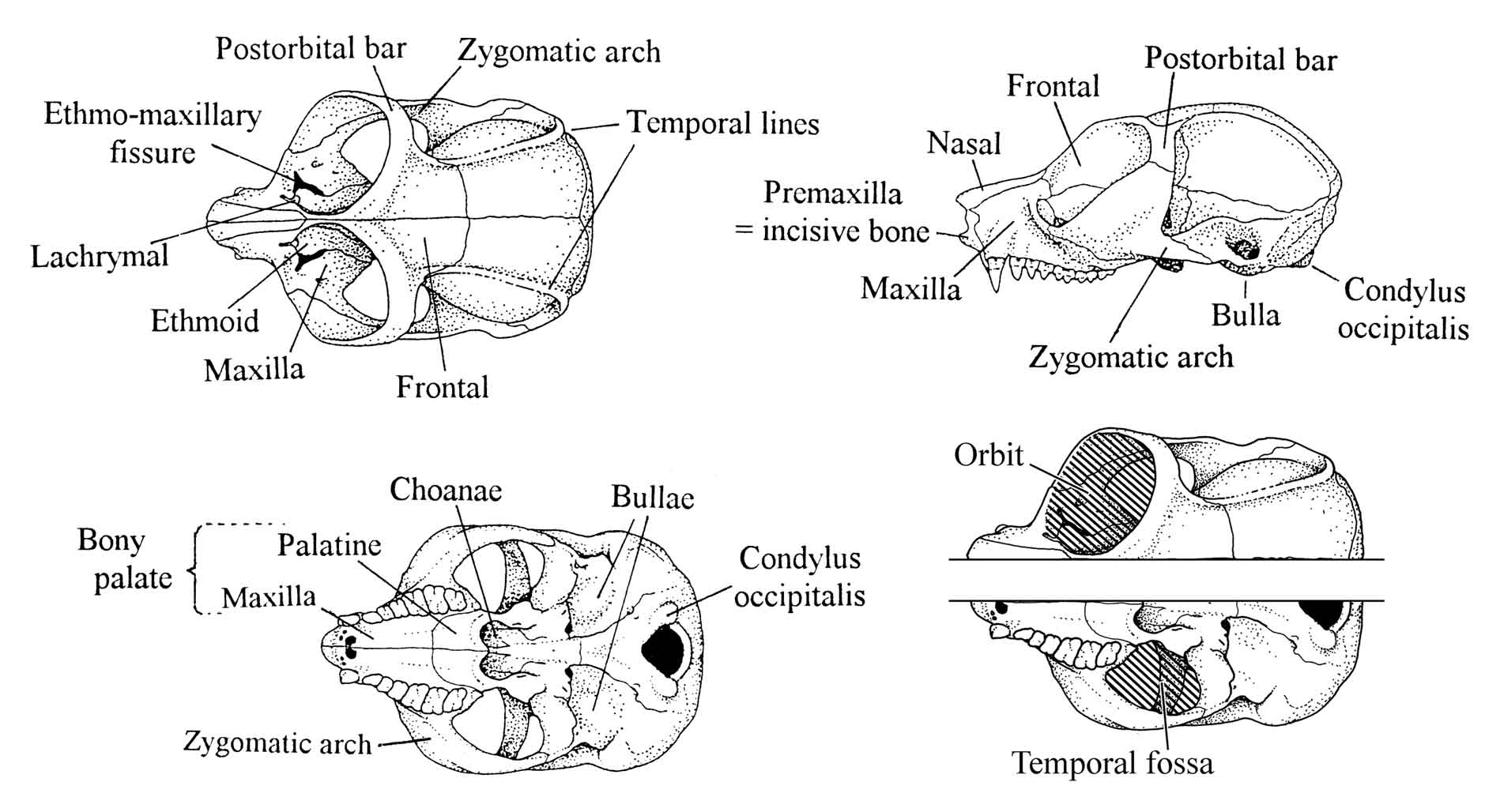
Figure 28: Skull morphology of the slender loris.

70
|
Management
of Lorises in Captivity. A Husbandry Manual for
Asian Lorisines (Nycticebus
& Loris ssp.)
Edited by: Helena Fitch-Snyder and Helga Schulze. Compiler: Lena C. Larsen |
Last
amendment: 2 January 2003
|