| Home |
|
|
Husbandry Manual for Asian
Lorisines
(Nycticebus & Loris ssp.)
| REPRODUCTION |
|
| Determining Gender |
|
| Adolescent Development |
|
| Estrous Cycles |
|
| Gonadal Morphology in Males |
|
| Copulation |
|
| Gestation |
|
| Parturition |
|
| Reaction of Others to Newborns |
|
| Resumption of Estrus |
|
| Interbirth Interval |
|
| Reproductive Seasonality |
|
| Litter Size |
|
| Infant Development and Parental Behavior |
|
| Captive Reproductive History |
|
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
REPRODUCTION
Slender Loris
The penis and clitoris of lorises are
similar
in size, shape, and color, which complicates gender determination.
The
slender lorises can be even harder to sex because their testes are
sometimes
partially or completely inguinal. The size and location of their
testes
vary. The vagina is often covered with fur in young females, and
close
examination is often necessary for safe determination. The tip of
the slender
loris’ clitoris shows a gray patch (Meier et al., in preparation).
The
clitoris is smooth with the exception of hairs on the tip, as
shown in
Figure 13. The penis is covered with fine, velvet-like hairs that
are barely
visible.
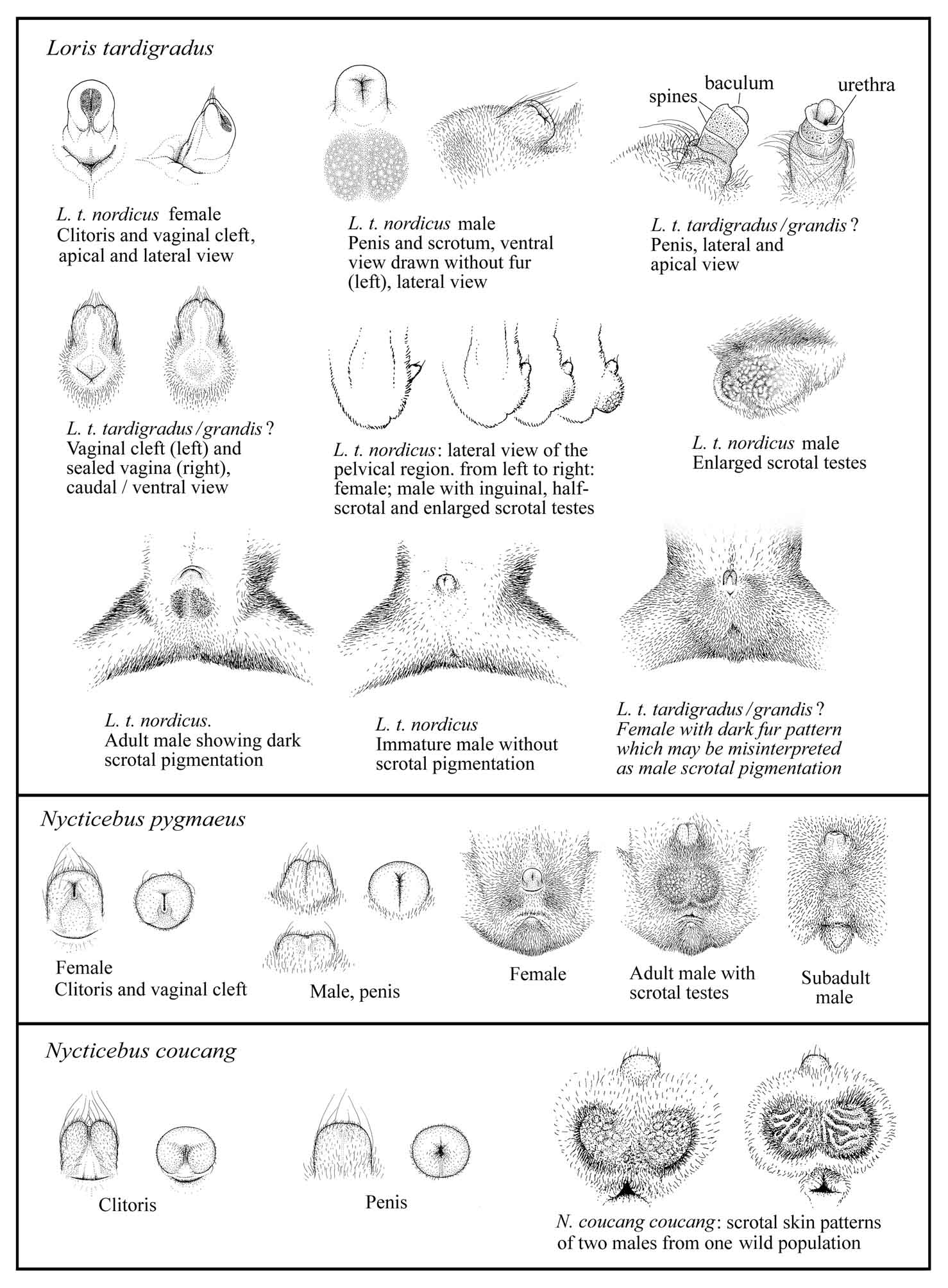
Figure 13: Discrimination of sex in lorises
28
29
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
Table 5: Studbook data of
parental
age in months at time of birth of first offspring.
|
Slow loris
|
Pygmy loris
|
Slender loris
|
||
|
Females
|
Mean
|
38.8
|
28.5
|
37.2
|
|
Standard Deviation
|
17.8
|
9.3
|
20
|
|
|
Range
|
22 - 75
|
19 - 37
|
17 - 102
|
|
|
number in sample (n)
|
17
|
4
|
18
|
|
|
Mean
|
50.6
|
33.3
|
35.1
|
|
|
Males
|
Standard Deviation
|
26.8
|
5.5
|
12.3
|
|
Range
|
21 - 98
|
27 - 37
|
17 - 53
|
|
|
number in sample (n)
|
14
|
3
|
13
|
Estrous cycles in prosimians range from 30 to 40 days. A mean cycle length for the slow loris is 42.3 days, with a range of 37 to 54 days. Captive slender lorises have a mean of 33.6 days with a range of 29-40 days (S.D. = 4.5 days, n = 7). L. t. nordicus estrous cycles are observed to have a mean of 42.8 days (n = 30), with a range of 32 to 67 days (Meier et al., in preparation). At the San Diego Zoo, a study of six female pygmy lorises during two years (n = 12) revealed that estrus lasted between 6 and 11 days (Jurke et al., 1997).
During much of the dioestrus interval, the external genitalia are largely obscured by fur drawn close in around them. The whole clitoris is pale and its central tract relatively flat; the labium, which is pressed against the basal area of the clitoris closing the vaginal cleft, is relatively small, flat and pale. At full estrus a considerable reddening and enlargement is evident, involving the central tract of the clitoris and the labium, all of which are taut-skinned, shiny, and strongly flushed. In addition, the labium is drawn down away from the base of the clitoris so that the vaginal opening is at its greatest lateral elongation, width, and depth (Manley, 1966).
Females should be closely monitored for signs of estrus: frequent vocalizing and the enlargement and reddening of the vaginal area. It is advisable to introduce males at the beginning of estrus and closely monitor the pair to confirm copulations and detect aggressive behavior. This will protect the female from possible injury and give accurate data concerning paternity and predicted dates for parturition. Cycle rating codes and a sample data sheet are provided in the appendix of this document.
A comparison of slow and pygmy loris testicular volumes indicate that there is no significant difference between the two species (Fitch-Snyder and Perez, 1989). These are unexpected results, considering the difference in body size. It is possible that the large testes could have evolved to produce large amounts of sperm during restricted breeding periods. When all the females are
30
Loris Husbandry Manual
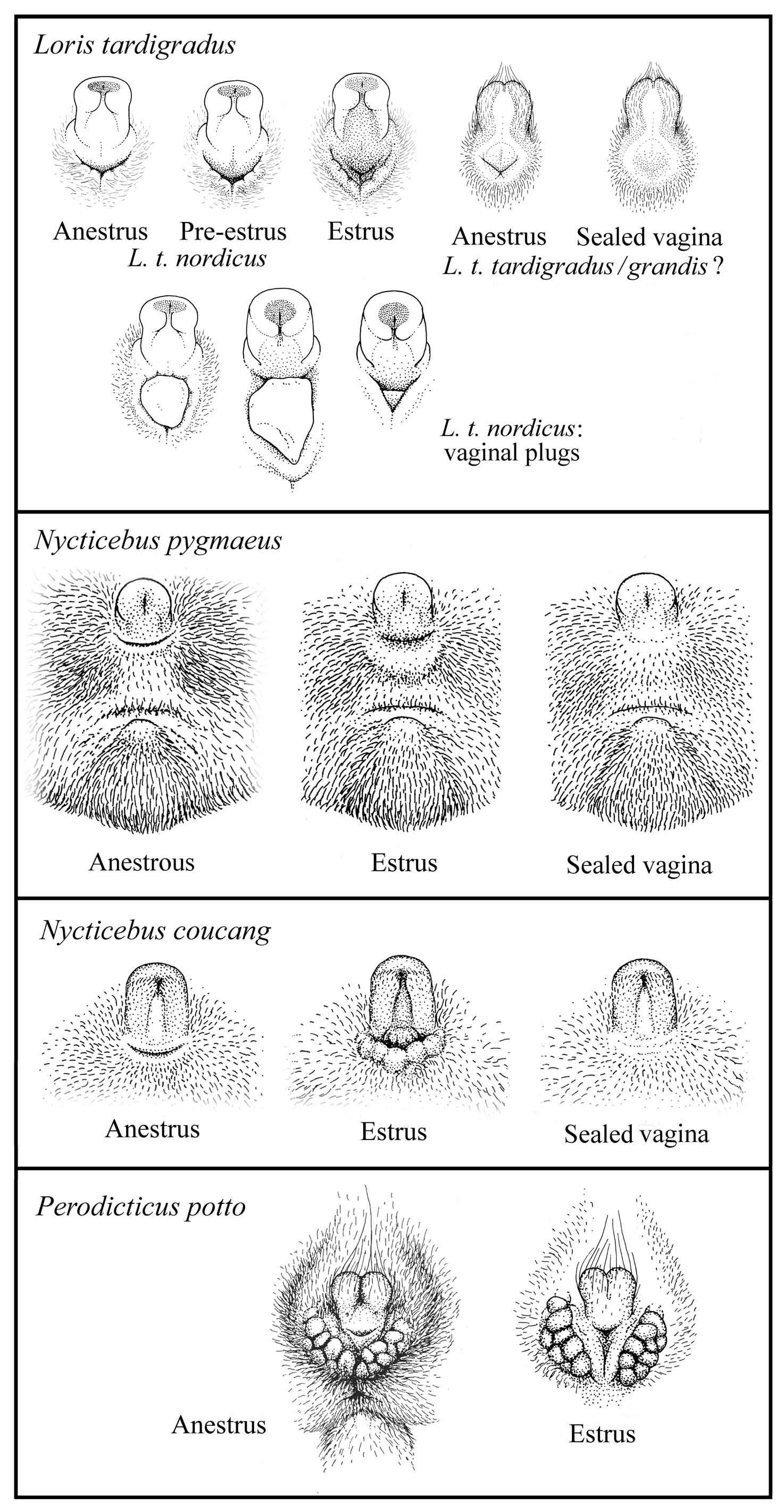
Figure 14: Signs of estrus in lorises and pottos
31
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
seasonally fertile, they would need to be capable of inseminating more than one female within a short period. Besides having disproportionately larger testes than the slow loris, pygmy lorises undergo changes in the pigment and texture of their scrotum during breeding season, thus making larger testes more conspicuous. The more conspicuous testes may be a factor that is selected for by estrus females.
Charles-Dominique (1977) found that potto males that occupied territories that overlapped with home ranges of females had bigger testes than those males that did not. In captive lorises, there does not appear to be any significant effect on testicular volume based on whether or not the male is housed with a female.
In slender lorises, the testes never descend into the scrotum at any time of the year (Ramaswami and Anand Kumar, 1965). A study of 151 slender lorises that were collected in the forests around Bangalore, India, showed that the testes are spermatogenically active throughout the year. However, there is a seasonal variation in the weight of the accessory glands; they reach the peak of activity in May prior to the period of mating in June-July (Ramakrishna and Prasad, 1967).
Slender Loris
The slender loris’ estrus lasts for a rather short time, perhaps less than 24 hours (judging by mating behavior and based on a study by Izard and Rasmussen (1985), who never observed that the cytological criteria for estrus persisted more than a day). During this time, copulation occurs in multiple bouts. The female urine marks; the male follows her around and urine marks or urine washes over the area where the female’s scent is present. L. t. nordicus males follow the female with appeasing vocalizations and repeatedly try to sniff her genital region. The female may answer his vocalization, and duets can sometimes be heard. The male’s urine washing, in this context, appears to be a displacement behavior indicating excitement. When ready for copulation, the female increasingly shows locomotion suspended upside down. The male mounts the female dorsoventrally, grips the female’s hind limbs with his feet and grips her midriff/torso with his forearms in order to support himself. The female supports them both during copulation. The copulation usually lasts for two to three minutes, although copulations lasting up to 16 minutes have been observed. The bouts can consist of multiple copulations interspersed with allogrooming and licking (Goonan, 1993; Izard and Rasmussen, 1985; Schulze and Meier, 1995b).
The males sometimes deposit a vaginal plug after successful copulation. The plug becomes hard and stearine-like. It is possible the plug aids in keeping the sperm inside the vagina; it may also prevent other males from copulating with the female (Schulze and Meier, 1995b). (See also Figure 14.)
Slow and Pygmy Loris
Slow and pygmy lorises copulate in a manner very similar to the slender loris, although they also incorporate “whistle” calls into their courtship. The female urine marks and whistle calls, intermittently stopping and turning her head to verify the male’s reaction. If he responds by sniffing, urine marking over her marks, whistling back and approaching her, she drops below a branch in a copulatory-invitation posture. The male then mounts the female in the same posture as the slender
32
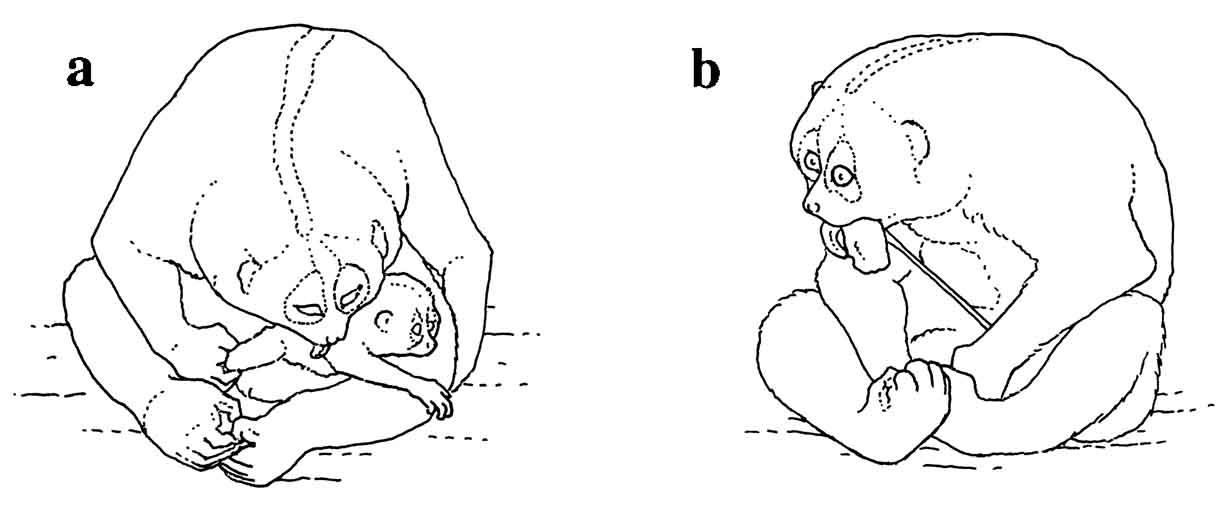 |
A slender loris
parturition was observed
at Duke University Primate Center. Contractions were
observed shortly before
dawn. The female began lickingF
|
igure 15: a) Female N. coucang licking the infant after giving birth, still in the labor and delivery posture; b) Female N. coucang eating placenta.
33
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
her vagina intensively and the actual birth occurred rapidly. The head, then the rest of the infant’s body, was expelled with two major contractions in less than a minute. The female received the infant with her hands and brought it up to her chest. The birth took place 20 minutes after dawn. The infant gained a grip on the mother’s fur after almost three minutes had passed. It was pink and almost hairless with the exception of fine yellow fuzz on the top of the head and the back. The orbits were bluish-gray and bulged disproportionally from the head. The mother licked it constantly and supported it with a hand for an hour after the birth.
Slender loris infants are relatively inactive for days after birth, especially in comparison to slow loris infants. This is not surprising in light of the fact that their gestation length is approximately one month shorter. Slender lorises are born at a much earlier developmental stage.
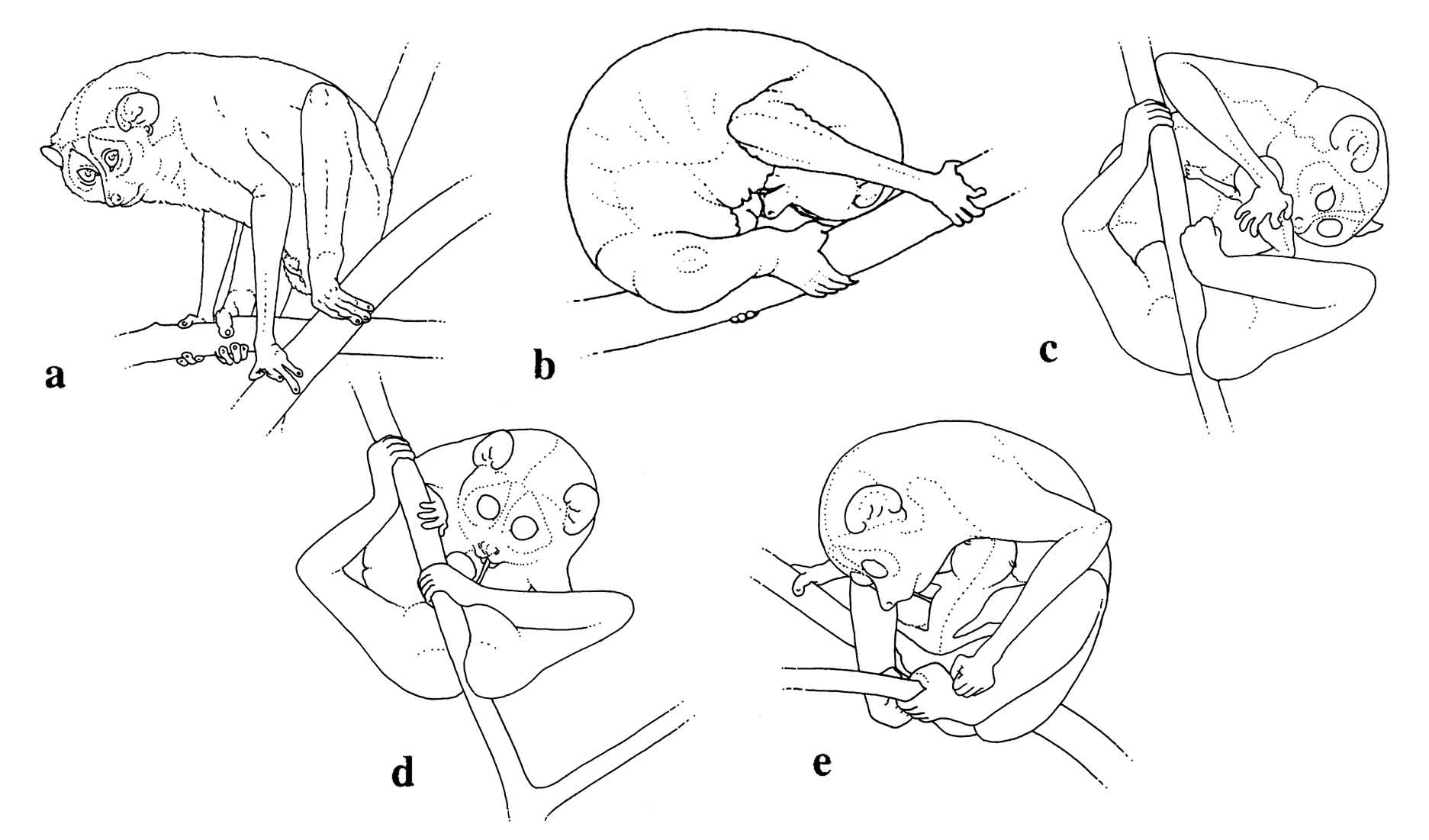
Figure 16: L.t. nordicus parturition: a) labor posture, b) licking during delivery, c) licking the baby, d) eating the umbilical cord, e) pulling and chewing the umbilical cord still attached to the neonate (a & b redrawn from photos by B. Meier).
Reaction of Others to Newborns
Although group rearing has been successful in many cases, it is important to identify pregnancies so that preparations can be made before parturition. If unsure about the reaction of the male, it is better to remove him before the birth occurs. The new mother may react aggressively towards the infant if she is stressed by the sudden removal of her cage partner. It is preferable to keep a group intact if they appear to get along, but it may not be possible to predict reactions of group members. In any case, the situation should be closely monitored.
Schulze and Meier (1995b) have found that slender loris pairs and families can remain together without conflict. However, new mothers occasionally become aggressive and fiercely defend their babies against curious conspecifics. The mother may allow her mate or older offspring to explore the
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
Table 6: Interbirth intervals
from
studbook (in months).
| . |
Slow loris
|
Pygmy loris
|
Slender loris
|
|
|
Previous Offspring
Survived
|
Mean
|
17.4
|
21.7
|
14.8
|
|
Range
|
6 - 53
|
13 - 30
|
6 - 42
|
|
|
Median
|
12
|
18.5
|
12
|
|
|
number in sample (n)
|
53
|
6
|
29
|
|
|
Previous Offspring
Did Not Survive
|
Mean
|
14.2
|
12
|
10.2
|
|
Range
|
7 - 59
|
9 - 16
|
5 - 24
|
|
|
Median
|
10
|
11.5
|
8
|
|
|
number in sample (n)
|
19
|
4
|
13
|
|
|
Without Regard to
Survival of Previous
Offspring
|
Mean
|
16.5
|
17.8
|
13.4
|
|
Range
|
6 - 59
|
9 - 43
|
5 - 42
|
|
|
Median
|
12
|
15.5
|
10
|
|
|
number in sample (n)
|
72
|
10
|
42
|
|
Slow lorises produce offspring throughout the year. Zuckerman (1932) examined 146 pregnant females that were taken from the wild. Pregnant females were found in every month but July, and there was a slight increase in pregnancies during September through November. In captivity, slow loris females also cycle more often during the latter half of the year (Izard et al., 1988), and it appears that they preferentially conceive during this time. Relatively few slow loris births occur October through January (Figure 17). Pygmy lorises have a distinct breeding season in July to early September with births from early February to the middle of March (Feng et al., 1994). In captivity, over 75% of all pygmy loris litters in North America were born from January through April, with a sharp birth peak in February (Figure 18). Pygmy loris females have their estrus sometime between the end of July and the first third of October (Jurke et al., 1997). Captive slender loris births in North America have taken place mostly in April, May, and June (Figure 19).
36
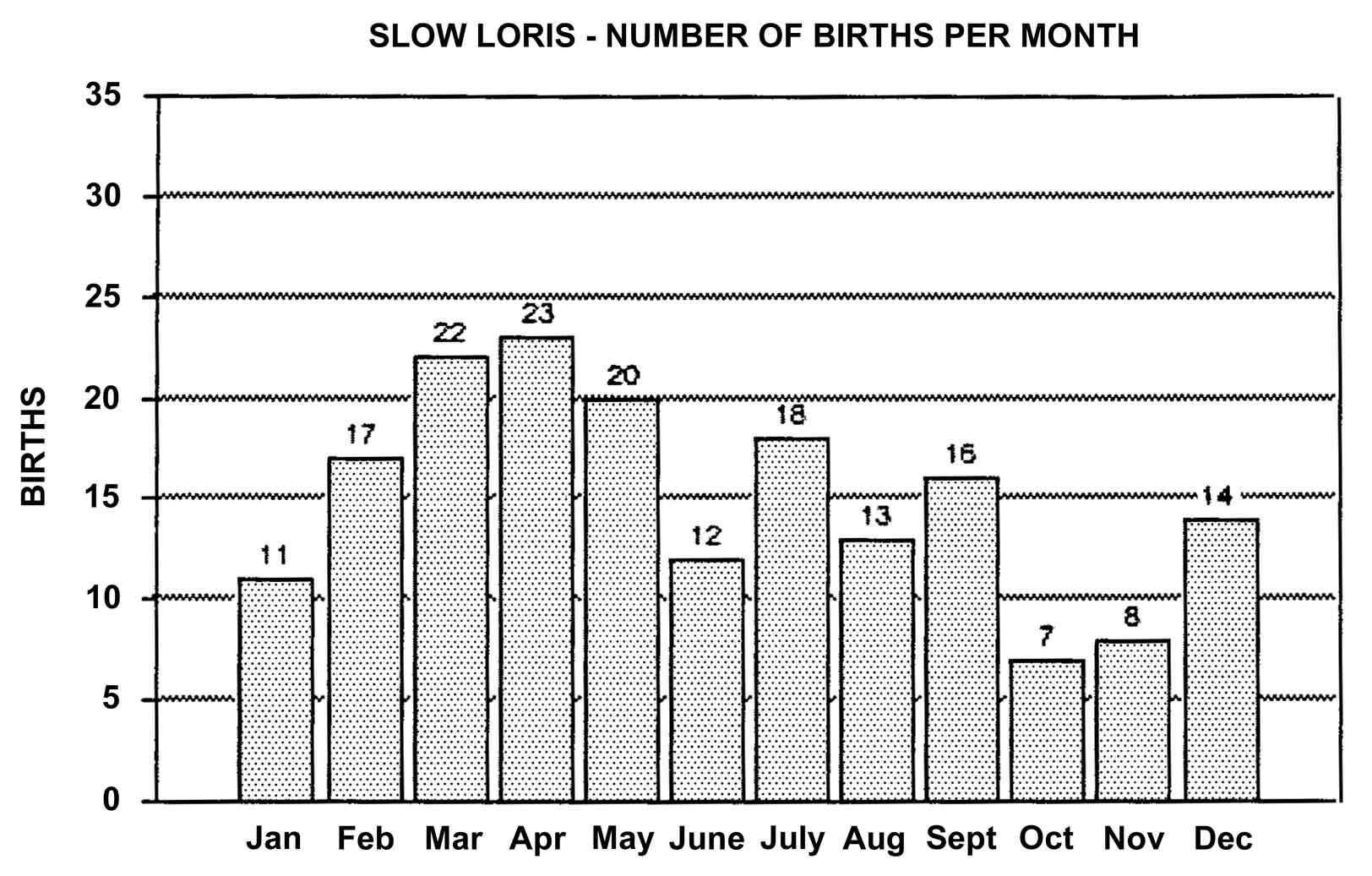
Figure 17: North American Regional studbook data of slow loris’ months of parturition (n=181).
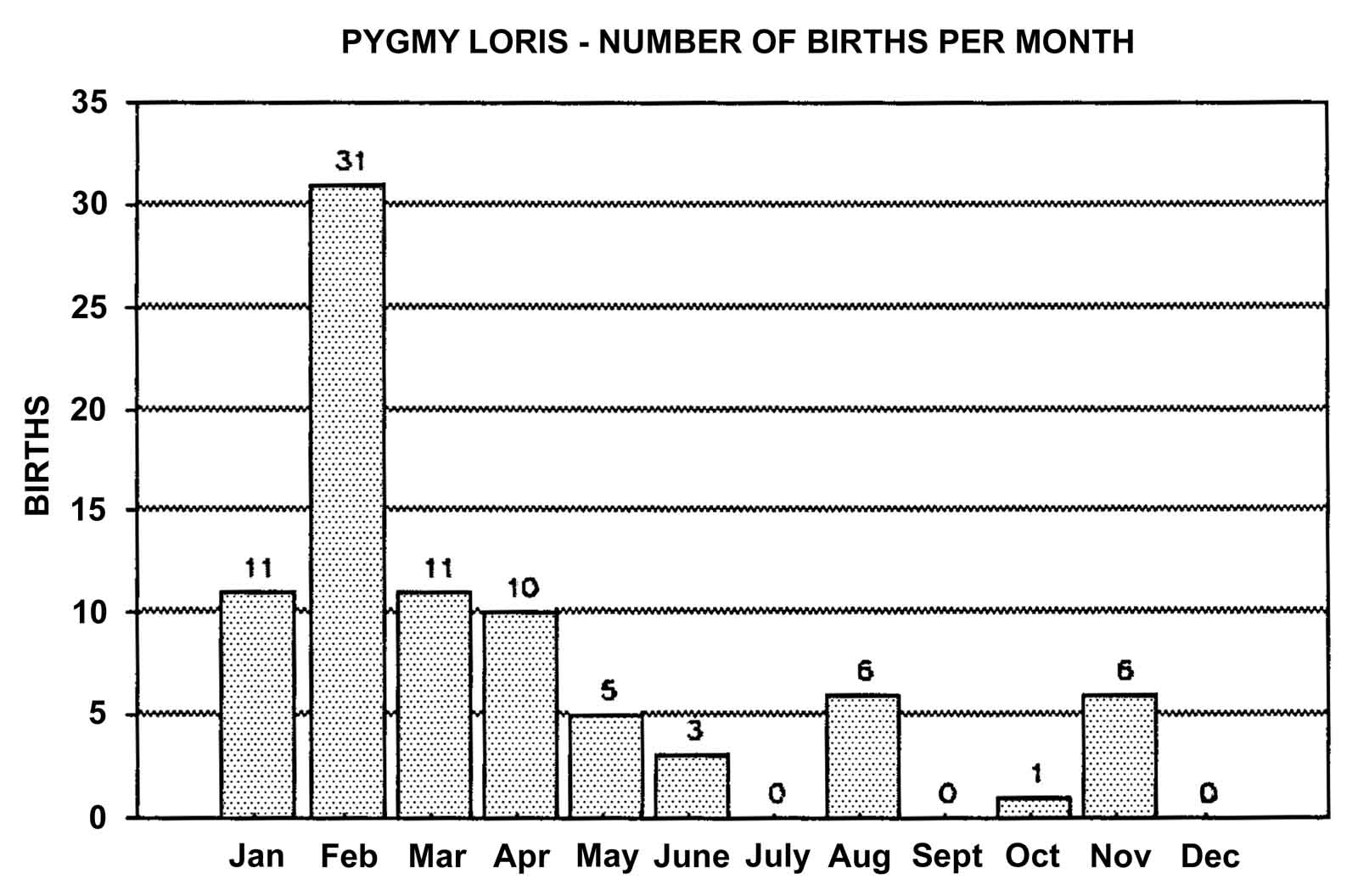
Figure 18: North American Regional studbook data of pygmy loris’ months of parturition (n=84).
37
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
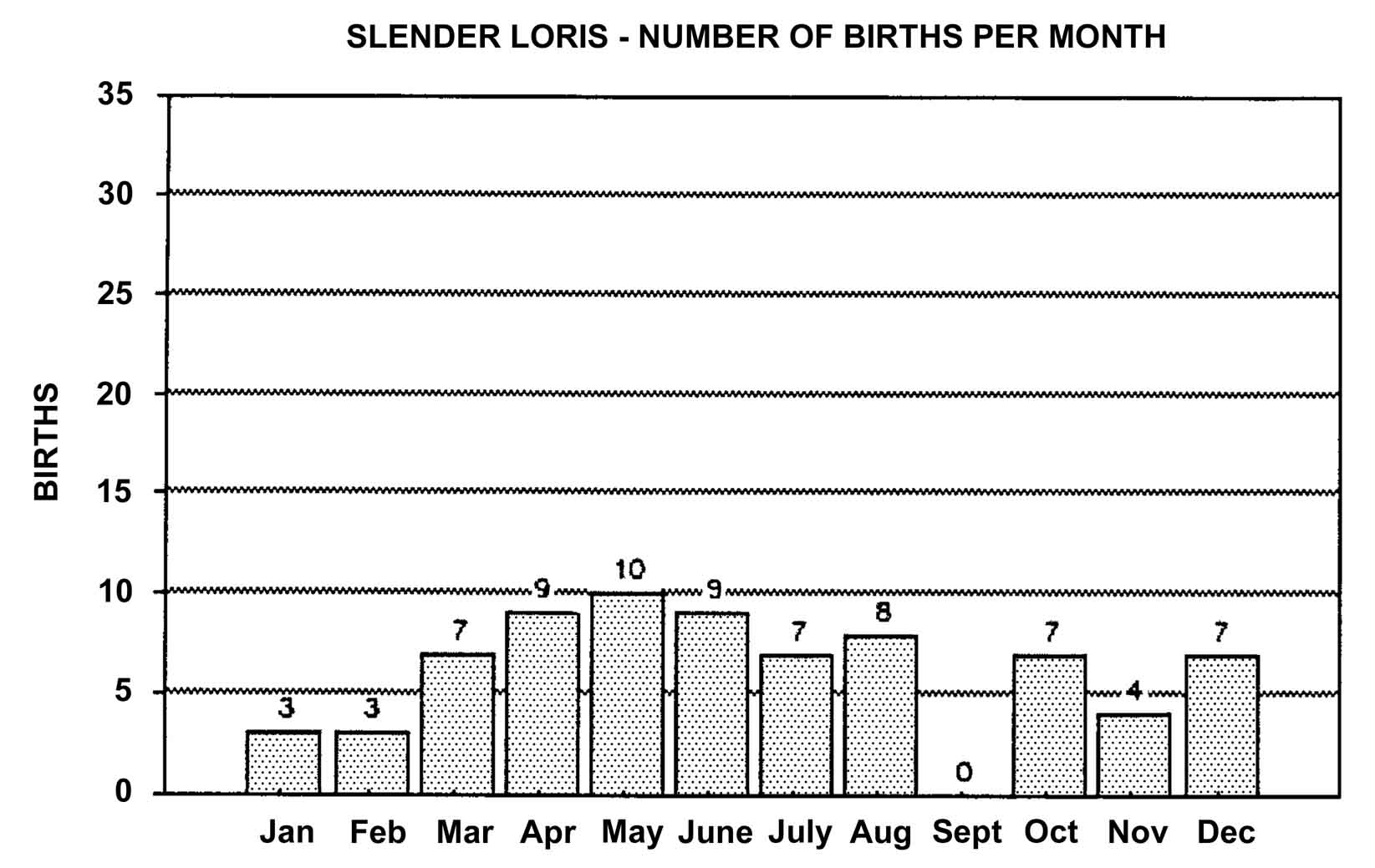
Figure 19: North American Regional studbook data of slender loris’ months of parturition (n=74).
Timing of estrus and birth may be influenced by environmental factors such as lighting. This may be especially true for the pygmy lorises, which show the greatest seasonal reproductive patterns of the three loris species. At the San Diego Zoo, pygmy lorises are kept on a natural lighting cycle. This is unlike the other breeding facilities in North America, which maintain their animals under artificial lighting with reversed day/night conditions. All 14 of the pygmy loris litters in San Diego were born from the middle of January through the end of March.
A long-term look at captive breeding through analysis of studbook data through 1997 shows that a total of 181 slow loris litters have been produced. Six of these were twins and only one pair survived past infancy. Slightly over 97% of all litters were singletons.
These data contrast with results from studbook analysis of the pygmy loris. Out of 56 litters produced, 55% of all pregnancies contained more than one fetus. There were 31 singletons, 25 sets of twins, and one set of quadruplets. Data from a captive breeding program at the Kunming Institute in China suggest that the rate of multiple births could be even higher than seen in North America. Nineteen litters have been born there, and in each case but one, litter size was two. The exception was a litter of three (Feng et al., 1994).
Number of offspring produced by slender lorises differs between subspecies. Studbook data of L. t.nordicus show twinning in approximately half of the births. Of 78 captive slender loris births in North America, only eight cases of twinning have occurred. Four of these cases were L. t. nordicus , while the subspecies in the other cases is unknown.
Chapman et al. (1990) have found that a diet of a large amount of insects in primates allows a
38
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
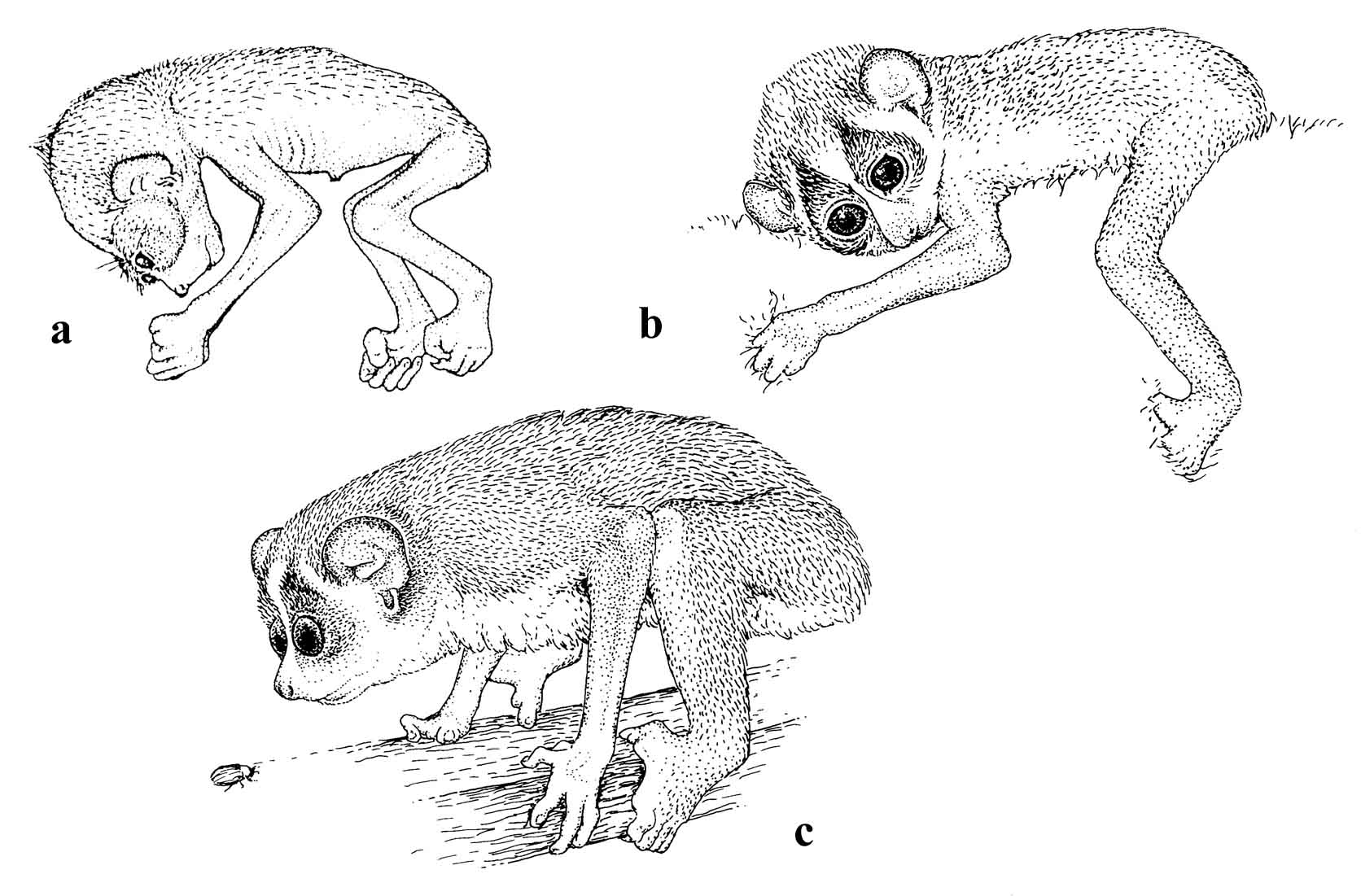
Figure 20: The early developmental stages of a slender loris (L. t. nordicus): a) neonate; b) 4 weeks old; c) 4 months old.
The time allocated to carrying and suckling differs between slow and slender lorises as the infants develop. A slender loris infant needs more care during its first month and is rarely parked. It also spends more time riding on the mother during its second month than slow loris infants do. As the slender loris infant grows older it develops rather rapidly. It discontinues suckling during nighttime when approximately four months old. The change from milk to solid food happens rather suddenly at age 70 to 80 days. Independence from the mother occurs after the fifth month. The initial slow development is made up by relatively rapid changes with dramatic shifts in behavior and time budget over short periods. The slow loris is weaned slowly over a longer period (Rasmussen, 1986a).
Bernhard Meier (unpublished) found that duration of the suckling period in single L. nordicus offspring (n=7) were approximately 148 days. Twins (n=2 pairs) grew more slowly, and their suckling period was 175 days. (See Figure 20.) This period is nearly identical to their gestation length (157-172 days, mean 162.3 days, n=14). Since females may have a postpartum estrus, they are often still lactating during pregnancy, and the infants are weaned shortly before the next birth.
The slow and pygmy loris infant is more independent of the mother and can be parked its first day after birth. Original duration of parking is not long, but the proportion of time spent parked increases with age. The development is more gradual in comparison with the slender loris. They maintain a close social relationship with the mother long after becoming independent. Slow lorises spend more time on social activities rather than solitary play, which appears to be more preferred by the slender loris (Rasmussen, 1986a).
Mothers are very responsive to infants click calls, and will immediately return to the infant if it vocalizes while it is parked. The social interaction between mother and offspring is predominantly composed of passive physical contact, followed by allogrooming directed from mother to offspring.
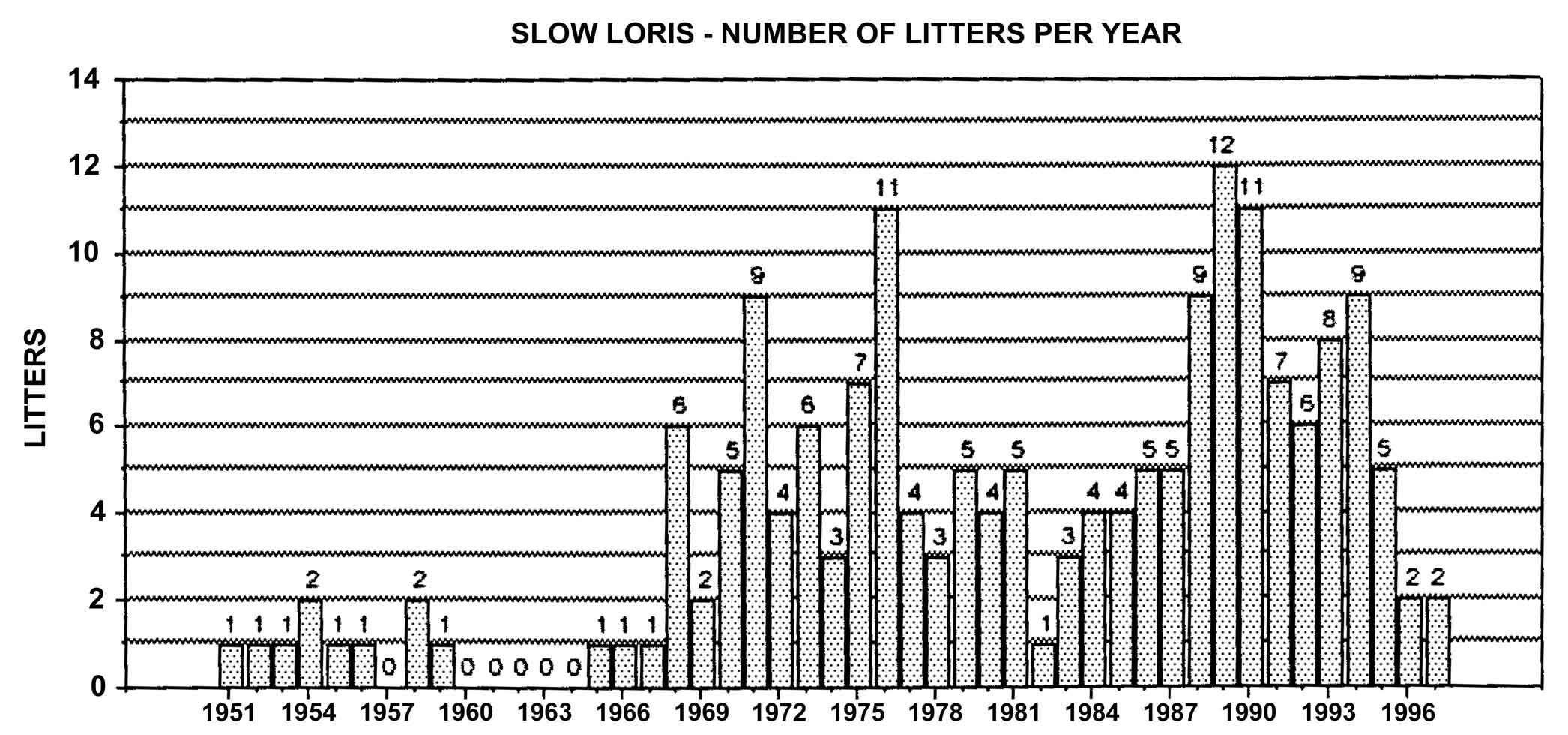
Figure 21: Slow loris births in North America, 1951-1998, n = 287
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
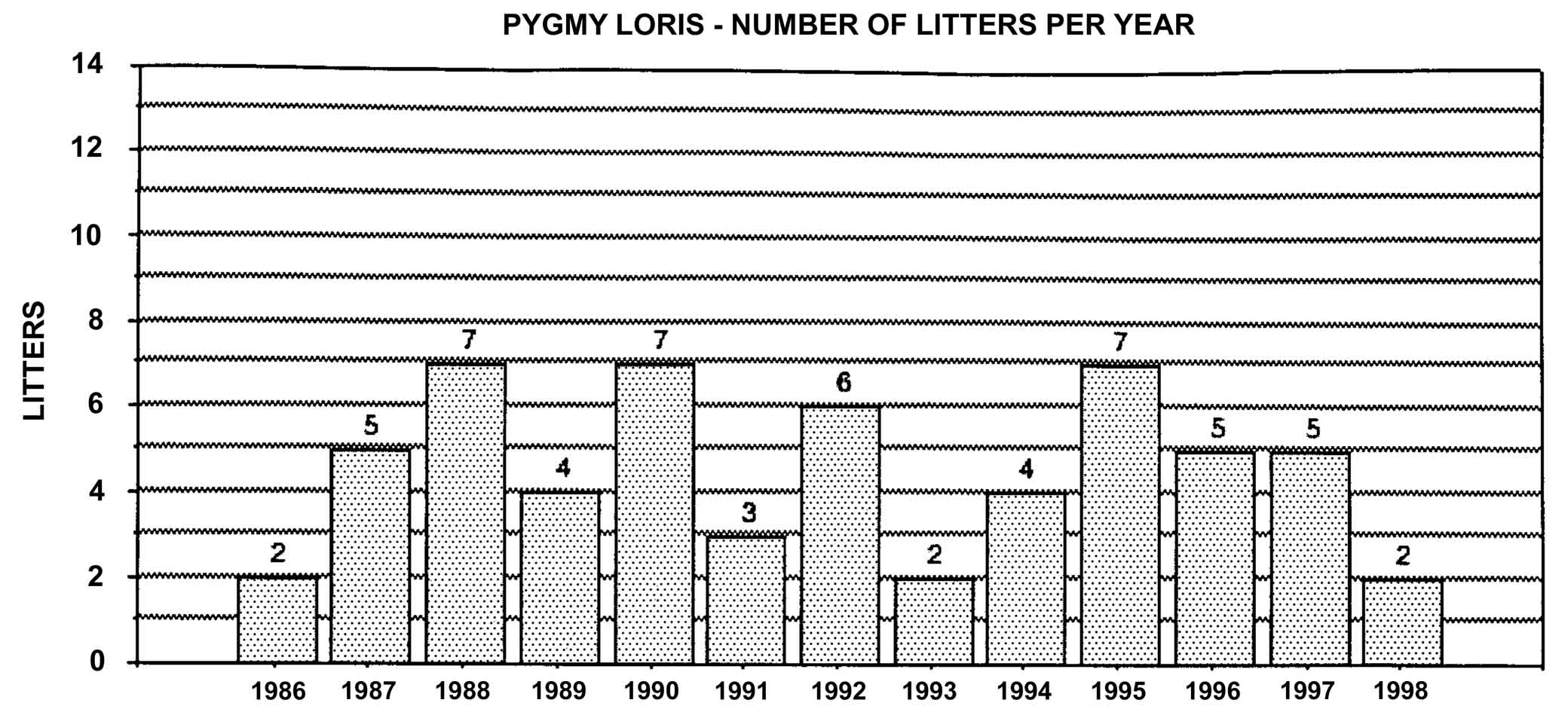
Figure 22: Pygmy loris births in North America, 1986-1994, n = 59.
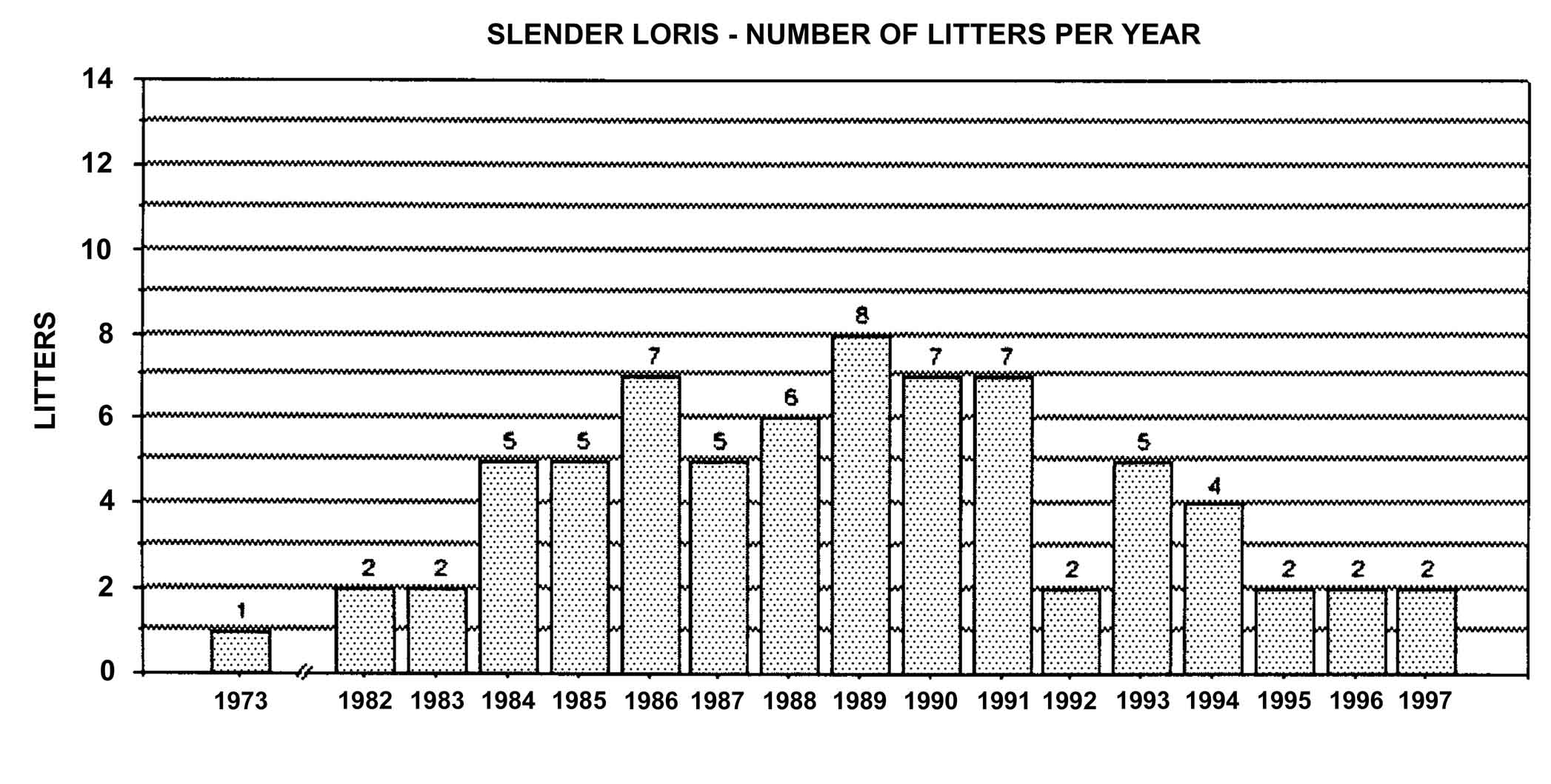
Figure 23: Slender loris births in North America, 1963-1994, n = 72.
Age-class fertility rates (Table 7) are based on slow and slender lorises’ whose exact ages are known. The pygmy loris data include breeders of both estimated and exact age, because the sample size is small (the exact age is only known for four of each gender).
|
|
|
|||||
|
Slow loris
|
Pygmy loris
|
Slender loris
|
||||
|
Females
|
Males
|
Females
|
Males
|
Females
|
Males
|
|
| 0-1 | ||||||
| 1 - 2 | .015 | .033 | .067 | .033 | .045 | .052 |
| 2-3 | .111 | .050 | .119 | .067 | .159 | .117 |
| 3-4 | .095 | .035 | .115 | .190 | .141 | .097 |
| 4-5 | .044 | .018 | .109 | .062 | .217 | .169 |
| 5-6 | .029 | .022 | .063 | .076 | .069 | .038 |
| 6-7 | .031 | .025 | .071 | .054 | .057 | .018 |
| 7 - 8 | .038 | .030 | .016 | .025 | ||
| 8-9 | .037 | .025 | .037 | .055 | ||
| 9 - 10 | .025 | .031 | .100 | |||
| 10- 11 | .009 | |||||
| > 11 | ||||||
* Fertility per age group is the sum of infants born to a given age group divided by the number of years lived by individuals of each sex in that group. (Data for older pygmy lorises are not available.)
Maximum fertility was reached at age two for the slow loris. Female pygmy lorises had the maximum fertility at the same age, while the male pygmy lorises lag a year behind. The slender lorises did not reach their maximum levels until four years old. The length of reproductive life differs between the slow and the slender lorises. However, the fertility rates shown is these figures may be misleading because it is not known if all the lorises in this analysis had equal opportunities to breed. Additionally, fertility rates in the pygmy loris are incomplete due to the fact that they have only been kept in North American zoos since 1987.
Table 8: Mean number of
litters produced
by successful breeders of known age (pygmy loris includes
individuals of
estimated age).
| Slow Loris | Pygmy Loris | Slender Loris | ||||
|
Females
|
Males
|
Females
|
Males
|
Females
|
Males
|
|
|
Mean Sd. Dev. Range n
|
2.1
1.2 1 - 4 17
|
2.8
1.6 1 - 5 14
|
1.7
0.8 1 - 3 15
|
1.6
0.7 1 - 3 15
|
2.6
1.9 1 - 6 18
|
2.7
2.6 1 - 9 13
|
The natality was the highest for the slender loris, with 46.2% of females reproducing at least once and averaging 2.6 (± 1.9 S.D.) litters by successful breeders (Tables 8 and 9 and Figure 24).
![]() Loris
Husbandry Manual
Loris
Husbandry Manual
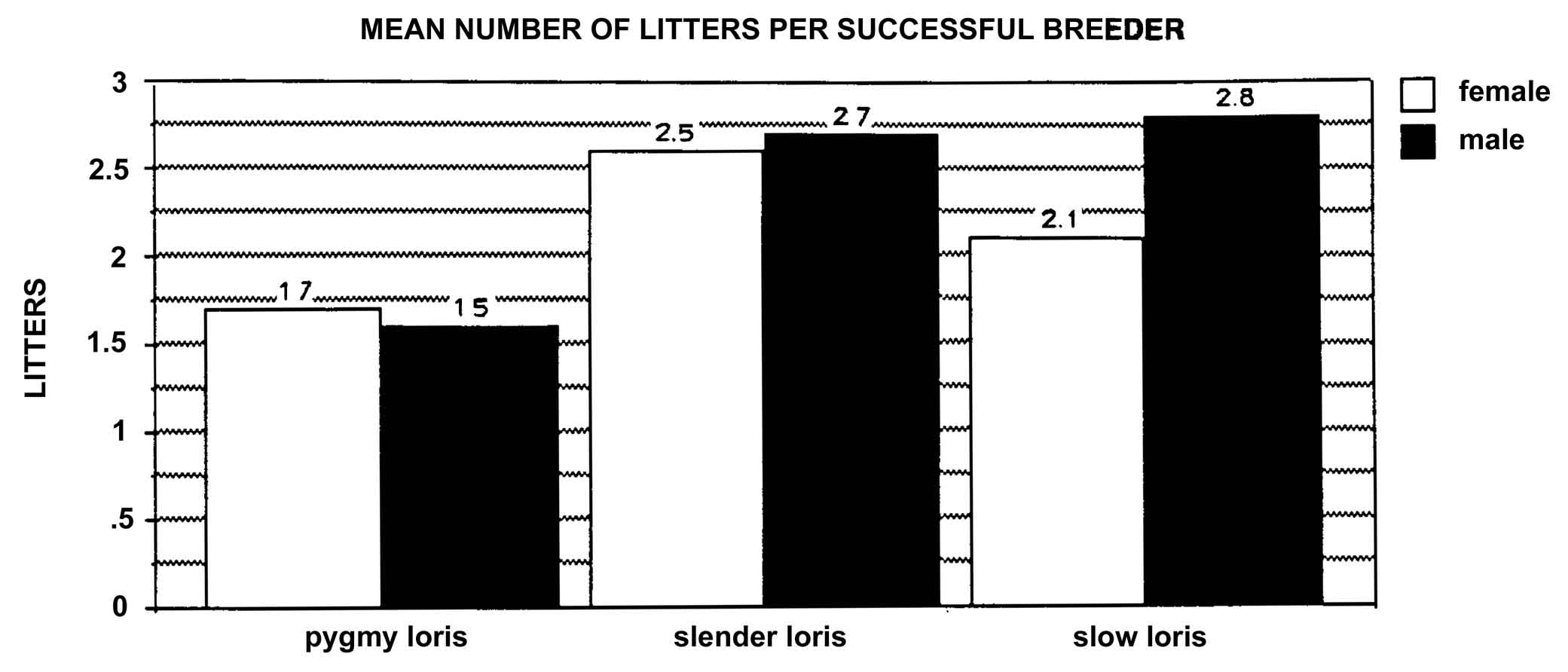
Figure 24: Mean number of litters produced by successful breeders of known age (pygmy loris includes individuals of estimated age).
Table 9: Litter production by
adults
| Slow loris | Pygmy Loris | Slender Loris | ||||
| Female | Male | Female | Male | Female | Male | |
| n | 40 | 54 | 10 | 15 | 39 | 34 |
| Breeders n
(%)
|
17
(42.5%)
|
14
(25.9%)
|
4
(40.0%)
|
3
(20.0%)
|
18
(46.2%)
|
13
(38.2%)
|
| Mean no.
of litters per
adult
|
.90 ± 1.32 | .72 ± 1.46 | .50 ± .71 | .27 ± .59 | 1.2 ± 1.82 | 1.1 ± 2.2 |
| Range | 0-4 | 0-5 | 0-2 | 0-2 | 0-6 | 0- 10 |
|
No.
of
litters per year of adult life
|
0.206 | 0.137 | 0.278 | 0.16 | 0.418 | 0.369 |
|
Management
of Lorises in Captivity. A Husbandry Manual for
Asian Lorisines (Nycticebus
& Loris ssp.)
Edited by: Helena Fitch-Snyder and Helga Schulze. Compiler: Lena C. Larsen |
Last
amendment: 2 January 2003
|