 |
 |
|
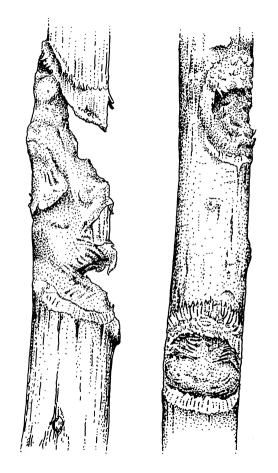
| Nycticebus pygmaeus gauge hole. Photo: Ulrike Streicher, Endangered Primate Rescue Center, Vietnam | Gauge marks of captive Nycticebus coucang (redrawn from a photo of branches in the collection of K.-H. Schweigert) |
| Home | Chapter
index
(captive care) |
|
|
|
Nutrition of lorises and pottos
Schulze, H. (compiler), with data by (in alphabetic order) H.
Fitch-Snyder,
B. Meier, K. A. I. Nekaris, K. Petry, F. Wiens et al.
Under construction; more data soon
See also online edition of the San
Diego
loris manual: nutrition
chapter
A cautionary note: experience at Ruhr-University
and information
from keepers showed that wrong or too abundant captive feeding
in lorises
easily leads to disease (such as dysbacteriosis, kidney lesions
or diabetes,
see below) and subsequent death with symptoms of wasting disease
(loss
of weight, malnutrition, sometimes changes of skin, hair loss or
other
problems). We recommend to strictly adhere to a certain daily
food quantity
regarded as adequate. The information below certainly needs
further improvement.
Regular health control with urine dipsticks for human use is
recommended
for early recognition of problems due to inadequate diet. Any
help for
improving this material, such as diets with which lorises have
been kept
healthy over several years, would be appreciated.
Content:
General informationDiet of loris and potto species
Slender lorises - basic information
Captive slender loris nutrition, examples: at Ruhr University, at Brookfield Zoo, at San Diego Zoo
Nycticebus pygmaeus - basic information
Captive pygmy slow loris nutrition, examples: at Brookfield Zoo, at San Diego Zoo
Slow lorises - basic information
Captive slow loris nutrition, examples: at Brookfield Zoo, at San Diego Zoo
Arctocebus - basic information
Perodicticus - basic informationFood-related problems in captivity
Nutritional deficiencies
Wasting disease in captivity
Treatment of wasting disease with Inulin
Other food-related health problemsFood items and food supplements: product information; see also page for nutrient content of food items.(in preparation, at present only empty page) and extra page about pellets.(under construction, preliminary)
Sorts of tree exudates:
Some addresses where to obtain food and food supplements
Review of nutrient content in food items, by Kathrin Petry: in preparationReferences
Some literature on the effect of inulinMilk composition, food during handrearing: see handrearing chapter
General information
All lorisids are more or less omnivorous and in their natural
habitat
feed on insects and other small prey - up to the size of small
reptiles
and maybe birds - and various vegetable food. They may have
specialized
on catching insect species other sympatric mammals will refuse, as
for
instance hairy caterpillars, very small, badly smelling or tasting
prey,
which means that they tolerate, but do not prefer such rather
abundant
food (Hladik, Hadik 1972; Charles-Dominique 1976). Recent field
studies,
however, showed that slow lorises of a Malaysian study population
have
specialized on a high-calorie diet consisting of floral nectar and
tree
sap (Wiens 1995, 2002)
Hladik and Petter (1970) observed that prey is found by systematic
searching of twigs and lianes with the nose close to the
substrate. In
captivity, the slender lorises at Ruhr-University find distant
prey mainly
with their eyes and by hearing it. Particularly the sound of small
objects
falling down attracts them immediately and causes quick pursuit.
Sniffing
and taste are employed for identification. Tests with various food
under
captive conditions showed that moving small prey is a favourite
food of
all lorisids. Even small living fishes, certainly no familiar
lorisid prey,
were taken, most probably because of their movements (Subramoniam
1957).
Dead, motionless prey is often rejected (Subramoniam 1957 and
observations
from Ruhr-University). Among vertebrates, smaller lizards and
geckos are
generally relished. Newly born mice or hamsters are eaten; freshly
killed
chickens, mice and rats were accepted; Kinnear (1919) observed
that captive
L.
t. lydekkerianus killed and ate two gerbils, and a slow
loris caught
a bat (Mackenzie 1929).
Dead birds were almost completely devoured by slender lorises,
only
one or two large wing feathers being left (Phillips 1931);
Catching and
eating of birds by slow lorises at San Diego Zoo has been observed
(Fitch-Snyder,
pers. comm.). Bird meat, dog-food and boiled eggs are taken by all
species,
but boiled meat and minced raw meat are frequently rejected
(Crandall 1964;
Blackwell, Menzies 1968; Kolar 1967, observation from
Ruhr-University).
Animal protein may also be given in the form of milk, baby formula
and
protein powder.
Feeding primates with vertebrate meat or baby mammals may cause
some
risks. Baby mice have some similarity at least to slender loris
neonates
who are small and initially partly naked, there might be a risk of
inducing
animals to cannibalism, and Montali and Bush (1999) warn that in
Callitrichidae
for instance feeding with baby mice may lead to infection with
lymphocystic
choriomeningitis virus, the cause of callithrichid hepathitis;
transfer
of other mammal diseases to primates might be possible. (Danger of
transfer
of bird diseases?)
In slender lorises, animal prey and immobile solid food are
fetched
with one or both hands with a fast and stereotyped movement which
seems
to be a fixed action pattern; sometimes in addition the teeth are
used.
Slender lorises immediately kill larger prey by crushing its head
with
the teeth; small and harmless animals are often eaten alive. For
transport
food particles are put into the mouth, leaving hands free for
locomotion.
Water is lapped up with the tongue in dog-like fashion. Lorisids
generally
seem to drink little water. Subramoniam (1957) observed that her
lorises
licked up tiny dew drops from the leaves. Our animals drink water
or fennel
tea only occasionally. More frequent drinking occured in the cases
of diabetes,
before birth and in cases of severe stress. Nutritious liquids
such as
milk or formula are either lapped up with the tongue by slender
lorises
or the animal makes the typical movement of seizing solid food ans
then
licks the liquid off the hand.
A slow loris was only once observed drinking; another one liked to
drink a cup of tea every evening (Mackenzie 1929). Sweet liquids
are taken
more readily than water. In a test with glucose solutions (5-40%)
a slow
loris preferred 20% solutions.
As the lower incisors and premolars of prosimians form a
specialized
toothcomb used for grooming their fur, some authors believed that
their
ability to bite off pieces of solid food is very limited. But in
fact,
the dentition is used to gnaw holes into tree bark for obtaining
sap (see
Nekaris 2000, 2001 and Nekaris, Rasmussen 2003 for slender
lorises,
Tan, 1994; Tan, Drake 2001 for
N. pygmaeus, Barrett 1984, Wiens
1995 and 2002 for slow lorises; Charles-Dominique 1977 and Oates
1984 for
pottos), lorises can bite very hard, for instance in defence, and
some
slender lorises managed to eat large pieces of apple offered to
them as
toys, but small food particles are preferred, maybe for
behavioural reasons
(typical prey-catching movement which also is an important play
behaviour).
Diet of loris and potto species
Slender lorises
In the wild, according to analysis of feces and stomach contents,
slender
lorises are extremely insectivorous, eating mainly very small
arthropods
(Hladik, Hladik 1972; Hladik, Petter 1970; Petter, Hladik 1970).
Feeding
on fruits, leaves, young sprouts and flowers was occasionally
observed
(Johnson 1984).
During an 10 month study of the socioecology of the slender loris
(Loris
tardigradus lydekkerianus) in a dry scrub jungle of Tamil
Nadu, India,
information on diet was systematically recorded. In 1173 hours of
direct
observation, 1238 feeding incidents were noted. Male and female
adults,
juveniles and infants were observed throughout the night or for as
long
as possible. The following variables were recorded: item eaten,
method
of capture, location while feeding, and size of substrate. With
the exception
of two geckos (probably Hemidactylus sp.), three seed pods
(Prosophis
juliflora), and gum, lorises ate almost exclusively
invertebrates,
including molluscs (slugs and snails) and arthropods (mainly
insects).
Orders of insects eaten include: Orthoptera, Hymenoptera,
Hemiptera, Coleoptera,
Lepidoptera, Isoptera, Diptera, Homoptera and Odanata. Lorises
almost always
grabbed prey with one hand, though flying prey was caught with two
hands.
Gum and sometimes ants were collected with the mouth. Sleeping,
cryptic
and colonial insects, along with snails and slugs, were picked off
branches.
Insects from the undergrowth, active insects and lizards were
caught by
stealth. Furious urine washing proceeded the consumption of some
beetles,
moths and ants. Vision and scent seemed to play the most important
role
in prey capture. Furthermore, insects were caught from all areas
of a tree,
though a preference was shown for insects on terminal branches and
middle
branches. The results of this study suggest that L. t.
lydekkerianus
ranks along with tarsiers as the most faunivorous primates. In
contrast
to previous studies of other lorisines, it does not appear that L.
t.
lydekkerianus specializes in cryptic and repugnant prey, but
utilizes
a broad spectrum of toxic and high quality insects. Finally, the
extreme
orbital frontality in lorises might be an adaptation to eating
insects
at close range (Nekaris 2000, 2001 and Nekaris, Rasmussen 2003; http://ssl.brookes.ac.uk/nocturnalprimates/wilddiet.html).
Nutritional analysis of a stomach content sample (1.52 g) from an
adult
female loris found dead: per 100g: total Ash = 400mg; Calcium =
72mg; Iron
= 49mg; Protein = 500mg; Fat = 1900mg.
Low basal metabolic rate possibly an adaptation to a fairly high
amount
of low quality food among the natural prey (Nekaris 2001).
Nycticebus pygmaeus
Weight changes: in summer much more slender, in winter fat
deposition
(U. Streicher, pers. comm and 2003 in press)
Fecal analysis from a pygmy loris caught in Vietnam contained 98%
insects
and 2% plant material (Fitch-Snyder, pers. comm; Fitch-Snyder,
Thanh 2002).
Gummivory in the wild has been observed, apparently including
stimulation
of exudate flow by gouging holes into trees (see information
of tree exudates below and figure;
many gouges
of about 1.8 - 2 cm with a depth of about 0.8 - 10 mm were found
in trunks
of two trees of the family Burseraceae in which pygmy
lorises were
observed (Tan, 1994; Tan, Drake, 2001). In captivity, at the Duke
University
Primate Center the characteristic gouges were found on wooden
substrate
(freshly cut branches of various sizes, particularly of
exudate-producing
trees such as red maple or sweet gum) (Tan, Drake, 2001). At San
Diego
Zoo lorises also produced gnaw marks on wooden substrates, and
there is
evidence that N. pygmaeus can detect gums embedded in
wooden blocks
(Tan, Drake, 2001, quoting Fitch-Snyder, pers. comm.). Gum, sap
for sale:
see below, addresses.
Slow lorises
Slow lorises are more frugivorous than Loris (Crandall
1964),
but also "ardent carnivores" (Tenaza and Fitch, 1984) who capture
insects,
small reptiles and perhaps birds. In Malaysia, during a field
study a major
part of the diet of observed lorises consisted of nectar, nectar
producing
parts of plants, plant sap and plant gum (a group of water-soluble
exudates
that seal wounds). 51.1 % of feeding observations were consumption
of nectar
/ floral parts of bertam palms Eugeissona tristis. Before
lapping
up considerable amounts of nectar, the lorises often bit off one
of the
three hard woody petals of the flowers. They also gouged holes
into the
superficial layer of the cambium of trees or lianae by using the
lower
anterior teeth,
lapped up the exposed saps and then quickly moved a few meters for
gauging
a new hole. Preferred trees were riddled with hundreds of small
holes,
and on thinner twigs sometimes the bark was chewed off from large
areas.
Solidified older gum was mostly collected by using the anterior
teeth as
scoops. For information about the behaviour see our enrichment
page. Amounts of available gum were usually large and
the lorises
spent up to 10 minutes at one site, but slimy, translucient,
reddish-brown
masses found in 55.3% of examined faeces may have been mucilage of
plant
gum, indicating that gums may be incompletely digested. (See information
about tree exudates). 30.2 % of feeding observations were
sap consumption.
3.6 % were gum consumption. Nectar could not be traced in faeces,
but 44.1
% of faeces contained flower parts indicating nectar eating; 51.1
% of
fecal samples contained pieces of bark and wood, probably from
gauging
holes or peeling off bark (Wiens 2002).
Other food items reported were live prey, including arthropods and
animals up to the size of small reptiles and birds (Wiens 1995 and
2002,
Barrett 1984) and fruits (according to Wiens, 2002, in Malaysia,
70.2 %
of faeces contained fruit remnants).
 |
 |
|
| Nycticebus pygmaeus gauge hole. Photo: Ulrike Streicher, Endangered Primate Rescue Center, Vietnam | Gauge marks of captive Nycticebus coucang (redrawn from a photo of branches in the collection of K.-H. Schweigert) |
In captivity slow lorises ate banana and other fruits, rice, dog-food, raw horse meat, insects, lizards, freshly killed chicken, mice, young hamsters and milk formula made of instant baby food with egg and honey and, as an addition, cod-liver oil and bone meal (Crandall 1964; Mackenzie 1929; Kolar 1967). According to Kolar (1967) lorises ate enormous quantities, for instance a whole banana, one chicken and 1/16 l milk formula per day.
Arctocebus (incomplete,
under construction)
Angwantibos eat about 85% animal prey, especially caterpillars, as
Charles-Dominique found out by examination of stomach contents.
Hairy caterpillars
are eaten after removing parts of the irritating hair by "massage"
with
both hands (Charles-Dominique 1976).
In captivity angwantibos eat various fruits, boiled rice, insects,
freshly killed mice, banana, minced raw meat, meat of birds and
small earth-worms
(Crandall 1964; Kolar 1967, quoting G. Durrell).
Perodicticus (incomplete,
under construction)
Pottos as an adaptation to dry season with limited food resources
develop
a thick fat layer during rain season, eating mainly insects and
fruits;
during dry season when food is scarce they are partly gum-eaters.
Their
long, sacculated colon and cecum may be regarded as an adaptation
to digestion
of the long-chained polysaccharides of gum. Pottos prefer slow
invertebrates
such as ants, beetles and snails they find on the surface of
branches;
they eat even poisonous and badly smelling species. If there are
enough
insects, they reject other food (Oates 1984). See also
Charles-Dominique
1976, 1977 for use of the dentition for gum consumption.
In captivity pottos are reported to be omnivorous, but erratic in
the
choice of food, eating banana, other fruits and vegetable, boiled
rice,
milk, hard-boiled eggs, dog-food, raw horse meat and newly born
mice. Minced
meat and insects were partly rejected (Crandall 1964). (But see
below:
nutritional deficiencies).
Slender loris diet at Ruhr-University Bochum:
Our lorises get the following diet per animal / day:
Recipe for milk formula for 10 slender lorises at Ruhr University bochum:
Diet may be slightly modified if necessary. If for instance old animals with kidney disease lose weight, they may get more mealworms
__________________________________
Loris diets at Brookfield Zoo (submitted by Carol Sodaro, changed)
| Slender lorises | Pygmy slow lorises | |
| ZPD (canned zupreem primate diet) | 12 g | 12 g |
| Fruit | 8.4 g | 11.2 g |
| Steamed Sweet Potato | 3 g | 6 g |
| Veggie | 6 g | --- |
| Leafy | 3 g | 4 g |
|
Dry Zupreem (dry biscuit
version of the canned
Zupreem diet)
Piece is a quarter of normal
zupreem piece,
not weighed
|
--- | 0 to 1 piece per bowl |
| Crickets | handfed | 2 pieces per bowl |
| Worms | handfed | 2 pieces per bowl |
__________________________________
Loris diets at San Diego Zoo: see San
Diego loris husbansdry manual
Food-related problems in captivity
Since too abundant or wrong feeding, particuarly in slender lorises, can apparently lead to a wasting disease with symptoms of diabetes and kidney problems. Urine dipstick tests at intervals are recommended to recognize such problems early and to adapt diet adequately. At Ruhr-University, animals are used to spreading of plastic foil in the cage floor for this purpose; urine can then be collected easily.
Nutritional deficiencies
Health problems due to insufficient nutrition in captivity have
been
reported. According to Manley (1966), they may partly be
recognized by
locomotor malfunctions. Carey and Carey (1967) describe a case of
osteomalacia
with limb deformation in a slender loris, caused by calcium
deprivation,
which was cured by giving more milk and additional vitamin D.
Blackwell
and Menzies (1968) report death of three pottos from
osteodystrophia, probably
because of too little calcium or too much phosphorus in the food.
(Their
diet consisted of various fruits, mostly banana, milk, hard-boiled
eggs,
newly-born mice and occasionally insects). Disturbances of
equilibrium,
partial paralysis and constipation in a young hand-reared potto
developing
at the age of 2.3 months could be cured with vitamin B added to
the food
(Rahm 1960). (In Microcebus murinus, according to Perret,
1982,
ten cases of lower-limb paralysis (flaccid paralysis) were
possibly caused
by lack of B factors and hydromineral (Ca, Na, K) disturbance). In
old
animals with wasting disease, animals suffering from malnutrition
because
of inadequate diet and anmals suffering from severe othe disease
cases
of changes of the skin (unnormal pigmentation, partial hair loss,
dry or
sore skin) have occurred. In slow lorises in some cases such
symptoms could
be cured by improved diet with more vitamins and calcium. For
primates
with skin problems, in addition, an attempt to add omega fatty
acids (for
instance contained in linseed oil) to the food is recommended (J.
Feuerstein
2001, e-mail-Information from alloprimate mailing list)
Wasting disease in captivity
A rather common problem in Loris is occurrence of weight
loss
and signs of malnutrition. Osman Hill (1937, based on almost 100
animals
which survived captivity only for a few weeks to four years)
reported loss
of weight, pellagra-like condition, swollen hands and feet often
exhibiting
haemorrhages and loss of epidermis and shedding of hair from the
limbs;
no case was cured. Osman Hill believed these symptoms to be caused
by some
nutritional deficiency. Data collected at Ruhr-University however
indicate
that rather inadequately composed, possibly too abundant food in
Loris
may lead to disease with signs of diabetes, often with kidney
problems,
dysbacteriosis (Prof. Krüger, Veterinary Academy Leipzig, Germany,
pers. comm.) and weight loss in spite of plenty of food available.
Perret,
1982, reports similar problems in a breeding colony of Microcebus
murinus.
The data compiled at Ruhr-University include eight deaths with end
stage
of cystic kidney disease and ten cases of wasting disease for
unknown reason
(no postmortem report available) or without kidney disease
diagnosed. At
least three other deaths were due to diabetes, in one case too
much insulin
and enlarged Langerhans-islets were found. In addition, blood
glucose tests
in live Loris after too abundant feeding indicated
diabetes, and
urine dipstick tests in animals with signs of wasting disease
showed that
urine contained both glucose and protein.
Treatment of wasting disease with inulin
(based on results of a study by K. Petry for her thesis, will be
published
here later in more detail)
In animals suffering from problems with inadequate composition of
intestinal
flora because of inadequate captive diet, first 0.5 g inulin
powder (made
of jerusalem artichoke) per kg body weight was added to the milk
formula
daily for two weeks, then 1 g per kg body weight for two weeks; in
addition,
bacterial biopreparations (Bird Bene-Bac, E. coli filled
into insects)
were given for replacing normal intestinal flora
(recommendation
from Prof. Krüger, veterinarian academy Leipzig). After this
treatment,
in 12 of 14 animals with former dysbacteriosis intestinal flora
was rather
normal again. Lifelong supply of the animals with 0.5 g Inulin per
kg body
weight was recommended (Prof. Krüger, pers. comm.) Following this
treatment, a lifelong supply with 0.5 g Inulin was recommended
(tests confoirming
this are made. E. coli supply for restauration of
intestinal flora,
however, must be tried with some caution because habits like
urinewashing
or touching the food with the hands and later urinemarking on
branches
touched with the hands during locomotion may lead to infection of
the urethra,
particularly in animals with glucose in the urine. Addition of
inulin to
the food in addition has a positive effect on the kidneys because
a higher
amount of urea is excreted via intestine (Prof. Krüger, Dr
Plesker,
pers. comm.; publications)
Other food-related problems
Deaths from trichobezoars
were diagnosed in one Nycticebus and two Arctocebus
(Manley
(1966) and in two lorises at Ruhr-University; bezoars may develop
because
of normal grooming behaviour and subsequent swallowing of hair,
which usually
is found in faeces. In slender lorises, in one animal at
Ruhr-University
loss of weight, reduced food consumption, occasional coughing and
subsequent
chewing were observed before sudden death; the other loris showed
no symptoms
noticed by the keeper (both animals were rather old). Prophylactic
addition
of paraffin oil (H. Ribjer, pers. comm.) or of some product made
for bezoar
prophylaxis in cats (for instance Miturat Catlax) to the food at
intervals
may help to prevent such problems.
Among more frequently occurring problems, tooth problems due to tartar or root infection deserve mentioning; infected teeth may lead to disseminated bacterial infection and secondary disease (Sutherland-Smith, M.; Stalis, I., 1999). Development of tartar may be reduced by addition of some hard food items (for instance insects with hard chiniteous skeleton) which help to clean teeth, particularly if soft food items such as avocado or banana are given.
Food items and food supplements: product information
Sorts of tree exudates:
Sap (phloem sap, manna, sap), fresh, oozing from wounds on
trees
after gauging holes into the bark (From: http://www.hilltopanimalhospital.com/sugarglider.htm).
Gum: dried exudate from the injured bark of some tree
species.
Some gum is sweetish and apparently very nutritious, and can
sustain life
for days in the absence of other food (From: http://www.naturalhub.com/natural_food_guide_vegetables.htm).
It
has been suggested that high calcium content may make gum an
essential
component of diet of animals eating large quantities of insects,
which
contain little calcium (Barret 1984, quoting Bearder and Martin,
1980 b).
But gum-producing Albizia sp., mentioned in this slow
loris field
study, were introduced, not native at Sungai Tekam.
Honeydew: exudate produced by some sapsucking insects
(From:
http://www.hilltopanimalhospital.com/sugarglider.htm).
References (nutrition in general):
Barrett, E., 1984: The ecology of some nocturnal, arboreal mammals in the rainforest of peninsular Malaysia. Ph.D. thesis, Cambridge University.
Blackwell, K.; Menzies, J. I., 1968: Observations on the biology of the potto (Perodicticus potto, Miller). Mammalia 32: 447-451.
Carey, D. E.; Carey, E. E., 1967: Calcium deprivation and osteomalacia in a slender loris, Loris tardigradus (Linnaeus). Journal of the Bombay Natural History Society 63: 428-429.
Charles-Dominique, P., 1976: Ecology and feeding of five sympatric lorisids in Gabon. Pp. 131-150 in: Prosimian Behaviour, Martin, R. D.; Doyle, G. A.; Walker, A. C. (eds.), Duckworth, London.
Charles-Dominique, P., 1977: Ecology and Behaviour of nocturnal primates. Prosimians of Equatorial West Africa. Duckworth, London. ISBN: 0 7156 0983 1.
Crandall, L. S., 1964: Management of wild animals in captivity. Univ. of Chicago Press. (Pp. 74-82: Family Lorisidae. Lorises, pottos, and galagos).
Fitch-Snyder; Schulze, H.; Larsson, L. C., 2001: Management of Lorises in captivity. A husbandry manual for Asian Loridae (Nycticebus & Loris spp.). Center for Reproduction of Endangered Species, Zoological Society of San Diego, Box 551, San Diego, CA 92112-0551.
Fitch-Snyder, H.; Thanh, V. N., 2002: A Preliminary Survey of Lorises (Nycticebus Sp.) in Northern Vietnam. Asian Primates Vol. IIX No. 1 & 2.
Hladik, C. M.; Hladik, A., 1972: Disponibilités alimentaires et domaines vitaux des primates à Ceylan. (The food supplies and vital territories of primates in Ceylon). Terre vie 26: 149-215.
Hladik, C. M.; Petter, J. J., 1970: Le loris tardigrade. Observations de terrains effectuées à Ceylan. Science et Nature 101.
Kinnear, N. B., 1919: Notes on the Malabar slender loris, Loris lydekkerianus. Journal of the Bombay Natural History Society 26: 836-837.
Kolar, K., 1967: Spitzhörnchen und Halbaffen [Tree shrews and prosimians]. Pp. 277-331 in: Grzimeks Tierleben Bd.10, Kindler Verlag, Zürich. (German)
Mackenzie, J. M. D., 1929: Food of the slow loris (Nycticebus coucang). J. Bombay Nat. Hist. Soc. XXXIII (4): 971.
Manley, G. H., 1966: Prosimians as laboratory animals. Symposia of the Zoological Society of London 17: 11-39.
Montali, R. J.; Bush, M., 1999: Diseases of the Callitrichidae. Chapter 48, pp. 369-376 in: , 1999: Zoo & Wild Animal Medicine: Current Therapy 4. Fowler, Murray E; Miller, R. E. (eds.). W. B. Saunders, Philadelphia. (Hardcover, 747 pages)
Nekaris, K. A. I., 2000. The socioecology of the Mysore slender loris (Loris tardigradus lydekkerianus) in Dindigul, Tamil Nadu, South India. Dissertation, Washington University, Department of Anthropology.
Nekaris, K. A. I., 2001: Some Aspects of Feeding Ecology of the slender loris (Loris tardigradus lydekkerianus) at Dindigul District, South India. In: Loris homepage, nutrition chapter from the in: Loris in the wild, old address, http://www.nocturnalprimate.org/wilddiet.html, at present at http://ssl.brookes.ac.uk/nocturnalprimates/wilddiet.html. Seen: 15.07.01.
Nekaris, K. A. I.; Rasmussen, D. T., 2003: Diet of the Slender Loris. International Journal of Primatology. International Journal of Primatology 24 (1): 33-46
Oates, J. F., 1984: The niche of the potto, Perodicticus potto. Int. J. Primatol. 5 (1): 51-61.
Osman Hill, W. C., 1935: Breeding of loris in captivity. Nature 136: 107-108.
Osman Hill, W. C., 1937: Treatment of the slender loris in captivity. Loris, June 1937: 85-88.
Perret, M., 1982: Stress-effects in Microcebus murinus. Folia primatologica 39: 63-114.
Petter, J. J.; Hladik, C. M., 1970: Observations sur le domaine vital et la densite de population de Loris tardigradus dans les forets de Ceylan. Mammalia 34: 394-409.
Phillips, W. W. A., 1931: The food of the Ceylon slender loris in captivity. Spolia Zeylanica 16: 205-208.
Pollock, J. I., 1986: The management of prosimians in captivity for conservation and research. Pp. 269-288 in: Primates, The road to self-sustaining populations, K. Benirschke (ed.), Springer, New York
Rahm, U., 1960: Quelques notes sur le potto de Bosman. Bulletin de l´Institut Français d´Afrique Noire, series A 22: 331-341.
Streicher, U., June 2003 in press: Saisonale Veränderungen in Fellzeichnung und Fellfärbung beim Zwergplumplori (Nycticebus pygmaeus) und ihre taxonomische Bedeutung. [Seasonal variation in the fur marking patterns and colour in the pygmy slow loris],. Der Zoologische Garten. (German)
Subramoniam, S., 1957: Some observations on the habits of the slender loris, Loris tardigradus (Linne). Journal of the Bombay Natural History Society 54: 387-398.
Sutherland-Smith, M.; Stalis, I.: Review of loris clinical information and pathology data from the San Diego Zoo: 1982 - 1995. In: Fitch-Snyder, H.; Schulze, H.; Larsson, L. C. et al. (meeting edition published in 1999, update in press): Management of Lorises in captivity. A husbandry manual for Asian Loridae (Nycticebus & Loris spp.). Center for Reproduction of Endangered Species, Zoological Society of San Diego, Box 551, San Diego, CA 92112-0551.
Tan, Ch. L.; Drake, J., 2001: Evidence of tree gougung and exudate eating in pygmy slow lorises (Nycticebus pygmaeus). Folia Primatologica 72: 37-39.
Tenaza, R.; Fitch, H., 1984: The slow loris. Zoo nooz 57: 10-12.
Wiens, F., 1995: Verhaltensbeobachtungen am Plumplori, Nycticebus coucang (Primates: Lorisidae), im Freiland. Diplomarbeit im Fachbereich Biologie der Johann Wolfgang Goethe-Universität Frankfurt am Main (unpublished) (German)
Wiens, F., 2002: Behaviour and ecology of
wild
slow lorises (Nycticebus coucang): social organization,
infant care
system, and diet. Dissertation, faculty of Biology, chemistry
and geosciences
of Bayreuth University, February 2002
Some literature on the effect of inulin:
Delzenne, N. M.; Roberfroid, M. R., 1994: Physiological effects of non-digestible oligosaccharides. Lebensm. Wiss. u. Technol. 27: 1-6.
Niness, K. R., 1999: Inulin and oligofructose: what are they? Presented at the conference nutritional and Health Benefits of Inulin and Oligofructose, held May 18-19, 1998, in Bethesda, MD. Pp. 1402-1406 in the Symposium publication (guest editors: Milner, J. A.; Roberfroid, M.). American Society for Nutritional Science.
Sobotka, L.; Brátova, M.; Slemrova, M.; Manak, J.; Vizda, J.; Zadak, Z., 1997: Inulin as the soluble fiber in liquid enteral nutrition. Nutrition 13: 21-25. Elsevier Science Inc., USA.
Kleesen, B.; Sykura, B.; Zunft, H.-J.; Blaut, M., 1997: Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am. J. Clin. Nutr. 65: 1397-1402. American Society for Clinical Nutrition.
Rowland, I. R.; Rumney, C. J.; Coutts, J. T.;
Lievense,
L. C., 1998: Effect of Bifidobacterium longum and
inulin on
gut bacterial metabolism and carcinogen-indiced aberrant crypt
foci in
rats. Carcinogenesis 19 (2): 281-285.
| Loris and potto conservation database - disease / captive care | Last amendment: 11 July 2008 |